
 |
||||||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: How do you approach the sequencing of hormonal therapies
for postmenopausal patients with metastatic disease?
DR LOVE: How do you approach the sequencing of hormonal therapies
for postmenopausal patients with metastatic disease?
![]() DR BUZDAR: It all depends on the patient’s prior exposure to endocrine
therapy and her age. For a postmenopausal patient who has never received
adjuvant endocrine therapy, I believe the first choice now is an aromatase
inhibitor.
DR BUZDAR: It all depends on the patient’s prior exposure to endocrine
therapy and her age. For a postmenopausal patient who has never received
adjuvant endocrine therapy, I believe the first choice now is an aromatase
inhibitor.
Anastrozole (Bonneterre 2000; Nabholtz 2000) and letrozole (Mouridsen 2003) both have clearly demonstrated better antitumor activity than tamoxifen in the metastatic setting. With the aromatase inhibitors, a higher fraction of patients obtain control of their disease for a longer period of time, and the patients overall experience fewer side effects.
![]() DR LOVE: If a patient receiving an aromatase inhibitor as first-line therapy
had a response and then her disease progresses, what’s your usual second-line
therapy?
DR LOVE: If a patient receiving an aromatase inhibitor as first-line therapy
had a response and then her disease progresses, what’s your usual second-line
therapy?
![]() DR BUZDAR: You have two choices. You can go from a nonsteroidal aromatase
inhibitor to either a steroidal aromatase inhibitor or an estrogen receptor
downregulator like fulvestrant. Those are the two choices with significant
antitumor activity in this type of situation. Some patients will say, “I want to
receive a therapy that I can control.” Some patients want to receive therapy for
which they don’t have to do anything. You can administer an injection at the
regular intervals, and they don’t have to worry about taking something every
day to remind them of their cancer.
DR BUZDAR: You have two choices. You can go from a nonsteroidal aromatase
inhibitor to either a steroidal aromatase inhibitor or an estrogen receptor
downregulator like fulvestrant. Those are the two choices with significant
antitumor activity in this type of situation. Some patients will say, “I want to
receive a therapy that I can control.” Some patients want to receive therapy for
which they don’t have to do anything. You can administer an injection at the
regular intervals, and they don’t have to worry about taking something every
day to remind them of their cancer.
Tamoxifen would be a reasonable option in that type of setting. However, considering the safety profile of tamoxifen versus an aromatase inhibitor, overall I would say that the aromatase inhibitor has a better safety profile.
Tracks 4-6
![]() DR LOVE: Can you talk about the eligibility, design and results of the
EFECT trial?
DR LOVE: Can you talk about the eligibility, design and results of the
EFECT trial?
![]() DR BUZDAR: Data show that the steroidal aromatase inhibitors are active
in patients who have been previously treated with anastrozole or letrozole
(Lonning 2000). Phase II data demonstrate that fulvestrant also has antitumor
activity in patients who have received aromatase inhibitors (Ingle 2006).
DR BUZDAR: Data show that the steroidal aromatase inhibitors are active
in patients who have been previously treated with anastrozole or letrozole
(Lonning 2000). Phase II data demonstrate that fulvestrant also has antitumor
activity in patients who have received aromatase inhibitors (Ingle 2006).
We didn’t, however, have any Phase III data to show whether exemestane and fulvestrant had similar efficacy. So EFECT was a bold attempt to compare them head to head. It was a double-blind, randomized, placebo-controlled trial. Neither the patient nor the doctor knew which drug the patient was receiving (Gradishar 2006; [2.1]).
The results were not surprising. As third-line therapy, both drugs showed a modest degree of activity, and approximately one third of the patients obtained a clinical benefit. The benefit was identical between the two groups of patients (Gradishar 2006; [2.1]).
![]() DR LOVE: EFECT used a loading dose of fulvestrant (2.1). Is a loading dose
now standard with fulvestrant?
DR LOVE: EFECT used a loading dose of fulvestrant (2.1). Is a loading dose
now standard with fulvestrant?
![]() DR BUZDAR: We don’t know whether that is the best approach. We can only
say that it was the way fulvestrant was administered when we compared it
to exemestane, and their efficacy was similar. If I have to use fulvestrant in
clinical practice in this type of setting, I will use the drug as it was administered
in the protocol.
DR BUZDAR: We don’t know whether that is the best approach. We can only
say that it was the way fulvestrant was administered when we compared it
to exemestane, and their efficacy was similar. If I have to use fulvestrant in
clinical practice in this type of setting, I will use the drug as it was administered
in the protocol.

Track 9
![]() DR LOVE: What about resistance to endocrine therapy? We hear more
and more about the cross talk between HER2 and other pathways.
Anything along those lines that you think is clinically meaningful?
DR LOVE: What about resistance to endocrine therapy? We hear more
and more about the cross talk between HER2 and other pathways.
Anything along those lines that you think is clinically meaningful?
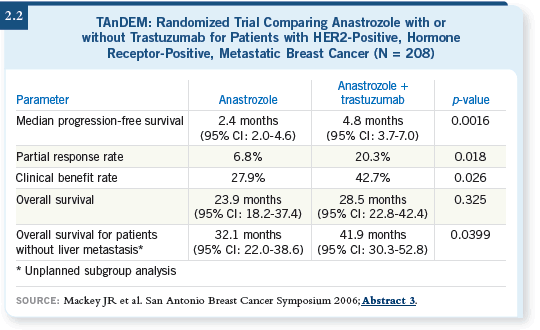
![]() DR BUZDAR: One study evaluating this question was the TAnDEM trial,
in which postmenopausal patients with estrogen receptor-positive, HER2-positive, metastatic disease received anastrozole alone or anastrozole and
trastuzumab. The patients who received the combination showed longer
control of the disease and a higher incidence of clinical benefit (Mackey 2006;
[2.2]).
DR BUZDAR: One study evaluating this question was the TAnDEM trial,
in which postmenopausal patients with estrogen receptor-positive, HER2-positive, metastatic disease received anastrozole alone or anastrozole and
trastuzumab. The patients who received the combination showed longer
control of the disease and a higher incidence of clinical benefit (Mackey 2006;
[2.2]).
Close to two thirds of the patients receiving anastrozole alone subsequently received trastuzumab, and we may not be able to evaluate the survival data. However, evidence suggested that patients receiving trastuzumab tended to have a slightly better survival, numerically (Mackey 2006).
![]() DR LOVE: Does this new information mean that patients like this should
generally start on trastuzumab and hormonal therapy?
DR LOVE: Does this new information mean that patients like this should
generally start on trastuzumab and hormonal therapy?
![]() DR BUZDAR: I believe these data are real. The TAnDEM trial did provide
a strong positive lead. I would discuss that approach with a patient who is
starting both therapies. We may be able to control the disease for a longer
period of time. Six months ago I would not have done it.
DR BUZDAR: I believe these data are real. The TAnDEM trial did provide
a strong positive lead. I would discuss that approach with a patient who is
starting both therapies. We may be able to control the disease for a longer
period of time. Six months ago I would not have done it.
Track 11
![]() DR LOVE: Can you provide an update of your neoadjuvant study for
HER2-positive disease that you presented a couple of years ago at ASCO?
DR LOVE: Can you provide an update of your neoadjuvant study for
HER2-positive disease that you presented a couple of years ago at ASCO?
![]() DR BUZDAR: Our study clearly demonstrated that close to 60-plus percent of
the patients receiving chemotherapy with trastuzumab showed a pathological
complete remission with no invasive cancer remaining in the breast or lymph
nodes (Buzdar 2005).
DR BUZDAR: Our study clearly demonstrated that close to 60-plus percent of
the patients receiving chemotherapy with trastuzumab showed a pathological
complete remission with no invasive cancer remaining in the breast or lymph
nodes (Buzdar 2005).
We recently updated that study, and we have treated another 22 patients with the identical approach of chemotherapy and trastuzumab. Of the 19 patients who were in the control group and received chemotherapy alone, three have experienced disease recurrence and one of those three has died (Buzdar 2007). Of the initial 23 patients assigned to chemotherapy and trastuzumab, not a single patient has experienced recurrence during three years of follow-up. All of those patients remain alive and free of disease (Buzdar 2007).
Among the 22 additional patients we treated with chemotherapy and trastuzumab, the pathological complete response rate was similar to our earlier experience (2.3). Also, that subgroup of patients still continues alive and free of disease. Of 45 patients we treated with trastuzumab and chemotherapy, we have not seen a single patient with a recurrence or clinical cardiac dysfunction (Buzdar 2007).
