| You
are here: Home: BCU
Surgeons Vol.2 Issue 3: Gershon
Locker, MD

Edited comments by Dr Locker
Implications of the updated ATAC trial data
I saw the initial data a month before it was initially presented in San Antonio
in
2001, and I was literally blown away. No one expected the trial to turn so
positive so quickly. My takeaway, even after the initial data, was that a newly
diagnosed, postmenopausal woman with hormone receptor-positive breast
cancer should be offered anastrozole, at least as an alternative, if not the
preferred treatment. In the year since, and with the updated data, my feelings
have not changed at all. The 47-month follow-up was very reassuring, because
the curves continue to separate. I would have been surprised if they didn’t.
Everyone is waiting for survival data, but it is important to remember the
disease-free survival is remarkably good — in the 88 to 90 percent range — in
this group of women. Therefore, it will be a while before we can evaluate
survival. However, it should be emphasized that in every adjuvant trial
demonstrating a disease-free survival difference, a survival difference has
eventually appeared.
I tell my patients that these data are preliminary, albeit with very strong
statistical support for efficacy. Approximately 75 percent of my postmenopausal,
ER-positive patients receive anastrozole instead of tamoxifen.
Anastrozole is a better hormonal adjuvant treatment than tamoxifen for ERpositive
postmenopausal women, but there will always be a subset of women
for whom tamoxifen may be preferred. For example, tamoxifen may be better
for women with osteoporosis coming in with the diagnosis of breast cancer,
particularly those already on bisphosphonates or calcitonin. In general,
I believe
anastrozole is the preferred treatment.
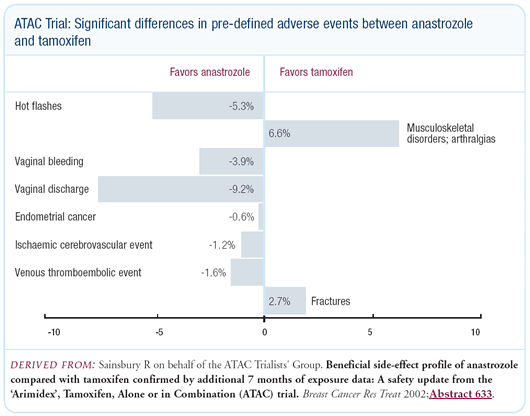
Risks and side effects of tamoxifen versus anastrozole
The biggest problem with tamoxifen is not the risk of thromboembolism or
uterine cancer, but managing uterine bleeding. Any woman who has uterine bleeding
on tamoxifen goes through a panoply of tests, which causes a great
deal of anxiety. A large percentage of women, sometime during their five years
of therapy, undergo a gynecologic procedure. This is what’s really unacceptable
about tamoxifen. We over-investigate some of these symptoms. This may be due
to our medical-legal milieu, but it contributes to a miserable lifestyle and
a lot of
anxiety for women on tamoxifen in the adjuvant and preventative settings.
Rates of breast-conserving surgery in the ATAC trial
There was a striking difference in breast conservation rates in the ATAC trial
between the two largest countries accruing patients — the United Kingdom
and
the United States. In a large, multivariate analysis taking every other factor
into
account, being an American woman increases your likelihood of having a
mastectomy by 44 percent, compared to being a British woman. There is
something about American patients or surgeons that seems to favor mastectomy
compared to what is done in the United Kingdom.
One potential explanation is that, although we have guidelines set by the
National Cancer Institute, American medicine is still individualized — the
surgeon and patient make the final decision. Guidelines tend not to be as
significant a factor in decision-making. Another issue is our American view
that
more is better. We have data, however, that this is not true in the mastectomy
versus lumpectomy decision. Psychological factors also play a role for some
women, in whom the thought of having a “cancerous breast,” even
if the cancer is removed, is not acceptable. It is also conceivable that geography
is a
significant issue for some women. In England, no woman is more than 50 or 60
miles from a major city. A woman in Montana may be hundreds of miles from a
center where she can receive radiation therapy.
We need to better educate surgeons and patients that there is no survival
difference between these two methods of treating early-stage breast cancer,
and
the preferred approach, when possible, is lumpectomy and radiation, for
aesthetic, psychological and a number of other reasons.
Clinical trials of aromatase inhibitors for risk reduction
Aromatase inhibitors have potential as chemopreventive agents. The data from
ATAC show that anastrozole is more effective in preventing contralateral breast
cancers than tamoxifen. It’s a natural transition to move anastrozole into
the
preventative research setting. I would have preferred that the European IBIS-II
prevention trial compare anastrozole to tamoxifen rather than to placebo.
Because it doesn’t, this trial would never fly in the United States, but
I’m glad
the NSABP-B-35 DCIS trial is making the correct comparison of anastrozole
versus tamoxifen.
Select publications
|
