|
You
are here: Home: BCU Surgeons
Vol 2, Issue 2: Monica
Morrow, MD
 |
 |
 |
 |
| Monica
Morrow, MD |
 |
Professor of Surgery
Northwestern University Feinberg School of Medicine
Director, Lynn Sage Comprehensive Breast Program
Northwestern Memorial Hospital |
|
 |
|
 |
Edited comments by Dr Morrow
The significance of micrometastatic disease
The increasing use of sentinel node biopsy has raised a whole
new set of questions, including whether micrometastases detected
by immunohistochemistry are clinically significant. This is a biologically
interesting question, and I strongly agree with the College of
American Pathologists’ consensus statement that we do not
yet understand the meaning of these micrometastases. The retrospective
studies of micrometastases have been a “mixed bag,” including
patients who have large areas of missed tumor in their lymph nodes
and patients with small numbers of cells in subcapsular sinuses
that aren’t even in the node parenchyma. It’s not particularly
surprising that some of these studies show no survival difference,
some show small survival differences, and some show very big survival
differences.
This is an area where both the NSABP-B-32 sentinel node study
and the American College of Surgeons Z-10 study will provide us
with very important information. Until that information is available,
we use immunohistochemistry only if there’s diagnostic uncertainty
on the basis of something seen on an H&E stain. We do not routinely
perform immunohistochemical staining of sentinel lymph nodes because
we don’t know what to tell the patients.
| AJCC
staging system revisions for lymph node micrometastases |
| “The sixth edition of the AJCC Cancer Staging Manual… records
an additional descriptor (i) for ‘ immunohistochemical’ in
cases that are histologically negative by H&E for lymph
node metastasis and in which IHC techniques were used. For
example, the designation pN0(i+) would indicate a case that
was H&E-negative but in which an isolated tumor cell deposit
not greater than 0.2 mm in greatest dimension was identified
by IHC. Likewise, the designation pN1mi(i+) would indicate
a case that was H&E-negative but in which a micrometastasis
greater than 0.2 mm but not greater than 2.0 mm in greatest
dimension was identified by IHC.” |
| SOURCE: Singletary SE et al. Revision
of the American Joint Committee on Cancer Staging System
for Breast Cancer. J Clin Oncol 2002;20:3628-36.
Abstract |
Clinical use of adjuvant aromatase inhibitors
The follow-up data with anastrozole from the ATAC trial look
good and suggest that the bone problems may be reaching a plateau,
which is encouraging. For women with low-risk, ER-positive, HER2-negative
breast cancers with very favorable prognosis, we still use as much
tamoxifen as anastrozole. For women with HER2-positive breast cancers,
I favor an aromatase inhibitor because of the debate as to whether
overexpression of HER2 predicts resistance to tamoxifen.
If the patient’s prognosis is less favorable, I would be
more likely to treat her with an aromatase inhibitor. There is
clearly a greater benefit from anastrozole compared to tamoxifen
in the short term. In a patient whose risk of relapse is quite
high, the absolute difference between these two treatments is much
larger. I would favor an aromatase inhibitor in this setting, and
the data we have right now in the adjuvant setting is with anastrozole.
Aromatase inhibitors for chemoprevention and
DCIS
The question about aromatase inhibitors as preventive agents
is a very important one. I am concerned that the IBIS-II trial — comparing
anastrozole to placebo — won’t give us the answer we
need. We’ll know if anastrozole is better than a placebo
but we won’t know how SERMs compare to aromatase inhibitors
or which is better in terms of overall health. We will not be able
to extrapolate these answers from two completely different study
populations, and this will leave us with another trial to do. In
addition, I would not recommend this trial to a woman at very high
risk. With tamoxifen on the market, proven to reduce breast cancer
risk, I don’t think taking a 50 percent chance of being randomized
to a placebo is a good choice. IBIS-II also has a randomization
for women with DCIS, but this compares anastrozole to tamoxifen.
I agree that treating DCIS is prevention — it’s a lesion
that carries a significantly increased risk of invasive breast
cancer. We tend to think of it differently because we treat it
like cancer, but the question is the same. The NSABP-B-35 trial
is asking the same question, randomizing women with DCIS to anastrozole
versus tamoxifen. It is a very good trial, addressing a very important
question, and I heartily support that study.

Management of the primary breast lesion in women
presenting with metastatic disease
I have traditionally thought that we should only treat the primary
tumor if it was progressing and causing local problems; however,
last year my colleague Seema Kahn and I published a study in the
Journal of Surgery of 15,000 women who presented with metastatic
disease from the National Cancer Database of the American College
of Surgeons.
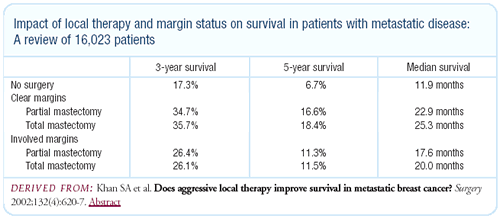
We looked at differences in survival based on surgical treatment
of the primary lesion versus no surgery. This was based on tumor
registry data. We controlled for a number of documented metastatic
sites and visceral versus soft tissue, and we found a very consistent
pattern wherein surgical treatment of the primary lesion was associated
with improved survival. While there may be selection bias to some
extent, the differences were seen in all subgroups.
This study raises some questions as we develop more effective
systemic therapy and keep people alive longer: Does it make sense
to reduce the tumor burden maximally so there are fewer places
the treatment has to work? We see this in renal cell carcinoma
for example, where removal of the primary tumor results in survival
differences. I think it’s an open question. However, removal
of the primary lesion is a reasonable option to try to maintain
local control and prevent morbidity, even if it doesn’t improve
survival. If the patient is clinically node-negative, I don’t
see that there’s a lot to be gained by dissecting the axilla.
Emerging
evidence of benefit from local control of the primary tumor
in patients with
metastatic disease |
| “…there is an emerging body of data that challenges
the previously held assumption that local control of a primary
tumor is irrelevant in the setting of metastatic disease. This
spans different organ sites (kidney, breast, stomach, colon),
and although much of this information comes from retrospective,
uncontrolled studies, there is a sufficient degree of consistency
to justify a prospective randomized trial dealing with this
issue.” |
| SOURCE: Khan SA et al. Does
aggressive local therapy improve survival in metastatic breast
cancer? Surgery 2002;132(4):620-7. Abstract |

Select publications
|
|
