| You
are here: Home: BCU Surgeons
Vol 2, Issue 2: Michael
Baum, MD, ChM, FRCS, FRCR
 |
 |
 |
 |
| Michael
Baum, MD, ChM, FRCS, FRCR |
 |
Professor Emeritus of Surgery
Visiting Professor of Medical Humanities,
University College London |
|
 |
|
 |
Edited comments by Professor Baum
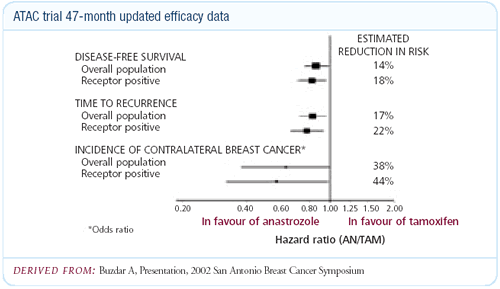
Updated data from the ATAC trial: 47-month follow-up
The new ATAC trial data gives me comfort and a sense of vindication
that we waited a year before starting to make therapeutic recommendations.
Last year I needed persuasion to use adjuvant anastrozole. It was
a nice option if tamoxifen could not be tolerated or was contraindicated.
This year, however, with the updated efficacy and safety data,
my position has changed. Now my default therapy for postmenopausal
women with estrogen receptor-positive tumors is anastrozole, unless
contraindicated. We have another year of follow-up in the ATAC
trial, and I am impressed by the separation of the curves. The
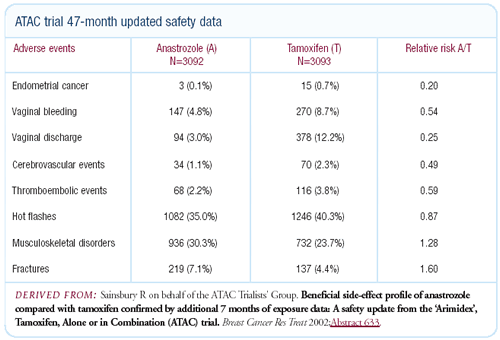
safety update is also comforting. The fracture rate isn’t
racing away, the relative risks are stable, and the other safety
profile issues continue to strongly favor anastrozole.
Clinical trials of anastrozole in the prevention
setting
Some might argue that the reduction of contralateral breast cancers
in ATAC looks less promising with the updated data than with the
original data — it has gone from about a 60 percent relative
reduction to about a 50 percent relative reduction in contralateral
breast cancer in the estrogen receptor-positive group. We had the
same experience early on with tamoxifen. The extremely dramatic
difference seen at three years was reduced over the next few years.
This suggests that these endocrine agents don’t prevent
cancer, but rather delay the appearance of cancer. Perhaps anastrozole
delays the appearance of breast cancer longer than tamoxifen. I
am confident that anastrozole will reduce the risk of estrogen
receptor-positive breast cancers — the adjuvant setting will
predict the preventive setting. The issue to me is the trade-off
and harm/benefit ratio.
Breast conservation rates in the ATAC trial
Gershon Locker presented breast conservation rates in the ATAC
trial at the 2002 San Antonio Breast Cancer Symposium. The dramatic
finding is that breast conservation is much less common in the
United States than in the United Kingdom and other countries. This
study shows the beauty of this incredible international database,
which allows us to explore cultural differences. It is fascinating
that the two countries with the highest rates of breast conservation
were France — which is not unexpected — and Brazil.
Brazilians are obsessed with the “body beautiful,” but
in addition, most Brazilian radiotherapists are trained in France,
so we see an interesting cultural issue.
In fairness to the Americans, we should not overinterpret these
data. The United Kingdom is a small country in which everyone lives
within 100 miles of a radiotherapy center. In contrast, parts of
the United States are thousands of miles from a radiotherapy center.
Radiation therapy consists of six weeks of treatment. I can sympathize
with women for whom it is just impractical to have breastconserving
surgery.
Intraoperative radiation therapy
This technology, in theory, could allow us to give all radiotherapy
at the time of surgery with a portable machine in a community hospital.
We have a neatly packaged mobile electron generator that delivers
X-rays at the tip of the probe. You can remove the tumor, apply
a spherical applicator to the tumor bed cavity, wrap the tumor
bed around this applicator and deliver radiotherapy to the index
quadrant. The whole process adds only one-half an hour to the operating
time.
This technique gives the biological equivalent dose of 50 Gy
to the tumor bed. The geometry is better than conventional radiotherapy.
Traditional conformal radiotherapy conforms to an uncertain shape.
With this method, we conform the cavity to the radiotherapy source,
so I think we'll do better than with conventional external beam
radiation therapy.
We did a Phase II study in 40 patients, and although I distrust
Phase II studies, it appears extremely safe and has excellent cosmetic
results. Only one woman developed ulcerated skin, which ultimately
healed. In this series, over the maximum four or five years of
follow-up, we have not had a single local recurrence.
We have opened an exciting trial, randomizing patients to conventional
postoperative radiotherapy versus intraoperative radiotherapy.
We’re hoping to enroll 2,000 patients in the study, so we
need to “spread our wings.” There is enormous interest,
and we have started randomization. We have groups in Australia,
North America and Germany.
Select publications
|
