| You are here: Home: BCU 8|2003: Eric P Winer, MD
Edited comments by Dr Winer
Treatment of patients with ER-positive metastatic breast cancer progressing on tamoxifen
In patients with hormone receptor-positive disease progressing on tamoxifen, one can switch to an aromatase inhibitor and there's a good chance the patient will respond. Three commercially available agents have been studied and are approved in this setting, so which agent to use is up to the individual oncologist.
Fulvestrant is also a good choice for these patients. In the two randomized studies comparing it to anastrozole, fulvestrant performed at least as well if not slightly better than anastrozole. Hopefully these patients would benefit from hormonal therapy for an extended period of time, and either fulvestrant followed by an aromatase inhibitor or the other way around would be reasonable alternatives.
Fulvestrant after progression on prior endocrine therapy
I'm concerned that physicians commonly give fulvestrant to patients with hormone receptor-positive metastatic disease who have received multiple chemotherapy regimens and hormonal therapies, and then judge fulvestrant to be a relatively inactive drug. This is probably not a fair evaluation.
In randomized trials of patients receiving fulvestrant or anastrozole in the metastatic setting, fulvestrant was at least as good as anastrozole, and I find the data quite persuasive. The one striking difference that favored fulvestrant was that there were fewer arthralgias and musculoskeletal complaints, and in our institution, the injection has not been a major issue.


First-line chemotherapy following adjuvant anthracyclines
Outside of a clinical trial, a woman who has received an anthracycline as adjuvant therapy could potentially receive docetaxel, paclitaxel, capecitabine or vinorelbine as first-line therapy for metastatic disease. In my opinion, the response rates for these agents are fairly similar. Some oncologists believe docetaxel is the most active agent, but I am not convinced that any of these agents have different activity. I tailor the treatment to the woman and base my decision on the types of side effects the woman would prefer to avoid.
Regarding toxicity, the best agents are probably capecitabine and vinorelbine. Alopecia is often an issue for women, and capecitabine is not associated with hair loss. If one is careful with the capecitabine dose, most side effects can be avoided. Over time, some women may experience chronic changes in their hands and feet, but that is the predominant toxicity encountered with capecitabine. In addition, I find when it's time for a patient to switch from hormonal therapy to chemotherapy, switching to capecitabine is not such a big step for them psychologically.
Capecitabine/docetaxel for metastatic breast cancer
I have occasionally given a patient capecitabine combined with docetaxel, and once the patient was stable, stopped the docetaxel. Fluid retention seen with higher doses of docetaxel makes it reasonable to stop the docetaxel and continue the capecitabine alone. I'm a little concerned about giving a combination for a short period of time, such as two months, and then moving on to capecitabine alone, because it's possible that giving very short courses of therapy may result in induction of resistance that may ultimately deny the patient benefits from the therapy.

Trastuzumab/chemotherapy combinations
For the time being, trastuzumab should not be given with an anthracycline because of the potential cardiotoxicity. The standard of care is trastuzumab plus paclitaxel, based on the FDA approval. Given the activity of docetaxel in women with metastatic breast cancer and the potential preclinical synergy, there are many physicians who administer trastuzumab plus docetaxel.
When we began studying trastuzumab plus vinorelbine in our first Phase II trial with about 40 women, the combination was well-tolerated and there was an overall response rate of approximately 70 percent. We then conducted a multicenter, Phase II trial of trastuzumab and vinorelbine in 55 patients and were again comforted by the safety and efficacy data.

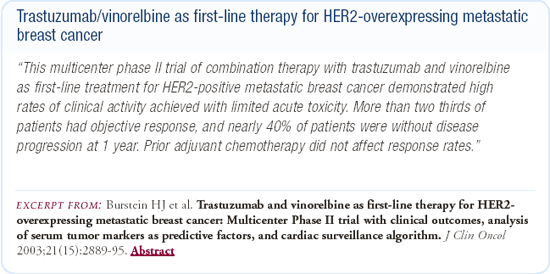
Phase III trial of trastuzumab plus a taxane or vinorelbine
There is an ongoing, multicenter, Phase III study involving about 50 sites in the United States comparing the combination of vinorelbine and trastuzumab with a taxane and trastuzumab regimen in the metastatic setting. The choice of which taxane to use, either weekly paclitaxel or weekly docetaxel, is left to the physician's discretion. Vinorelbine has not been one of the first-line agents in the treatment of patients with metastatic breast cancer, but we think that the vinorelbine and trastuzumab combination is a very promising regimen. If we want physicians to take this regimen seriously and consider incorporating it into an adjuvant program in the future, it has to be compared head-to-head with the taxanes.
Trastuzumab for patients with ER-positive, HER2-positive metastatic disease
I still strongly consider hormonal therapy in these women, however, there is suggestive evidence that patients with HER2-positive disease may be less likely to respond to hormonal therapy. For that reason, if I were on the fence about using hormonal therapy or moving on to chemotherapy, I would switch to chemotherapy more readily in patients with HER2-overexpressing disease.
When it is time to switch to chemotherapy in patients with HER2-positive disease, most of us believe trastuzumab is the standard of care. The question is whether to use trastuzumab plus chemotherapy or trastuzumab alone. I think in the United States, and certainly in my own practice, trastuzumab plus chemotherapy is more commonly given. The survival benefit with trastuzumab in the pivotal trial was seen when the combination of chemotherapy and trastuzumab was given up front. Also, there's a sense that response rates, and therefore control of tumor-related symptoms, are higher when chemotherapy is added to trastuzumab.
I don't believe there are many right and wrong choices in the treatment of metastatic breast cancer, but at this time, a woman who has metastatic, HER2-positive, ER-negative disease, who has never received chemotherapy, should receive a regimen that includes trastuzumab. I am impressed by the survival benefit seen with trastuzumab in Dennis Slamon's study. If anything, that survival benefit was minimized by the crossover in the group of women who didn't receive trastuzumab initially and by the fact that the HER2 assay was an imperfect assay.
Trastuzumab has actually changed the natural history of HER2-positive, metastatic breast cancer. We have women living far longer than they used to, and the unfortunate manifestation is that we are seeing an increasing number of patients with central nervous system metastases.
Nonprotocol adjuvant trastuzumab
I try not to use trastuzumab in patients with Stage II and IIIA breast cancer outside of a trial, because it's not an established therapy. In patients with inflammatory breast cancer, I don't know that we're ever going to have a randomized study, and at least 50 percent of the time the tumor is HER2-positive. I would be hard-pressed to criticize a physician who wanted to use a trastuzumab-based regimen in a patient with HER2-positive, inflammatory breast cancer.
I feel patients who are eligible for the randomized adjuvant trials should be encouraged to participate. Outside of those trials, I think that the standard adjuvant treatment is a non-trastuzumab-containing combination.
Importance of accurate HER2 testing
Whenever we have a new therapy requiring a predictive test, how that therapy performs is dependent on how good the test is at identifying the appropriate target. Both the NSABP adjuvant trial and the Intergroup trial indicated that HER2 testing in centers around the country — both community centers and academic centers — appeared to be less than perfect. Approximately 25 percent of the time, the test that was done in the local hospital — nonacademic institutions and academic institutions alike — couldn't be confirmed at a central testing site.
We need to be careful about where the HER2 testing is performed and view results from less-experienced labs with caution. This is especially important in the adjuvant setting where, unlike the metastatic setting, we have no way of knowing if the treatment is working, and we're committing the patient to a course of therapy.
Also, when we are banking on results from clinical trials, it is critical that we know the testing is accurate. Currently there's no established adjuvant role for trastuzumab, but I suspect in the next three to five years we'll learn whether it's an effective adjuvant therapy. Then accurate testing will be important to correctly identify the patients who will receive the maximum benefit from therapy.
When HER2 retesting is indicated
In metastatic disease when the initial HER2 test results and the clinical situation are inconsistent, one should consider retesting the patient. I've had a number of patients whose tumors were IHC zero, but their clinical presentation was consistent with HER2 amplification, so I retested. In each one of those cases there was not a discrepancy, but still I think it's worth doing. Even if I find a discrepancy only two out of 100 times, I'm doing those two patients a huge service.
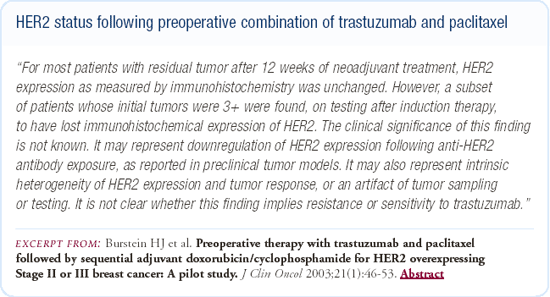
Changes in HER2 status following exposure to trastuzumab
We recently published a study of 40 patients with Stage II and III breast cancer who had HER2-overexpressing (IHC 2+ or 3+) disease and were treated preoperatively with a combination of trastuzumab and paclitaxel. Of the patients who had residual tumor and could be assessed postoperatively, approximately 20-25 percent had a change in the IHC status. While the numbers are extraordinarily small, it looks like the change in IHC status might be a little more common in patients who were initially 2+ rather than 3+; the IHC typically changed from 2 or 3+ to 0 or 1+.
We don't know exactly what is going on in these cases — perhaps it's just variability in testing or perhaps it's an effect of trastuzumab. We don't have FISH data on these patients yet, and it's possible some of these patients have FISH-negative tumors. Also, in our current study we have one patient in whom IHC and FISH testing has revealed a HER2-negative and a HER2-positive tumor side-by-side. Although most of us think of HER2 as more homogeneous than heterogeneous, it is possible that some patients have both types of tumor.

Select publications
|
