| You are here: Home: BCU 8|2003: Editor's Note
 |
Editor’s Note |
|
| Adjuvant Dilemmas |
For the last two years, our education group has produced a prostate cancer audio series for urologists and radiation oncologists. We quickly appreciated a dramatic contrast in the approach to adjuvant endocrine therapy compared to breast cancer.
Reassured by the sensitivity of PSA testing to detect early recurrence, urologists rarely utilize adjuvant androgen suppression after radical prostatectomy, even in very high-risk situations. The strategy of waiting for PSA relapse has never been validated by randomized clinical trials, and older, more classic, adjuvant approaches utilizing endocrine treatment immediately after local therapy resulted in survival curves remarkably similar to what was seen with breast cancer. Prostate cancer research leaders and community-based physicians are justifiably concerned about exposing men who might be cured with surgery to therapies with substantial side effects. However, most patients are unaware that a leap of research “faith” has been taken when endocrine therapy is delayed.
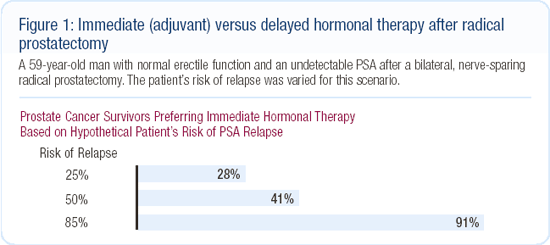
In September 2002, we gathered more than 300 prostate cancer patients and their guests for a one-day town meeting. We presented a variety of clinical scenarios and asked participants to vote — using anonymous keypad polling — on what their theoretical treatment preference might be based on the information presented. This event produced remarkable findings with regard to androgen suppression. When Dr Mark Soloway, a urologic oncology research leader, presented the option of adjuvant endocrine therapy, many patients and guests believed it to be a rational and preferable choice even though Dr Soloway did not support that approach (Figure 1).
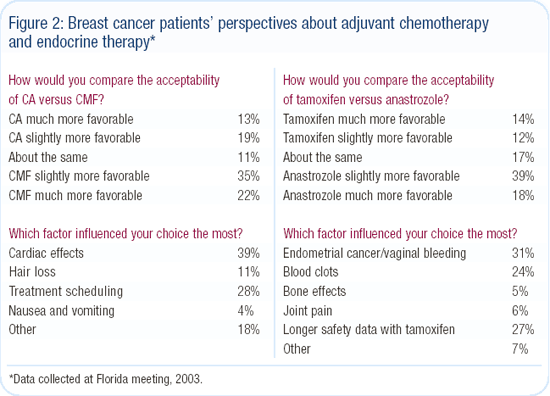
Compelled by the positive feedback and interesting data we received from this event, we conducted two similar “Breast Cancer Patient Perspectives Meetings” in New York and Florida this year. More than 700 breast cancer survivors and guests listened as a faculty of national research leaders presented a variety of common adjuvant case scenarios and described what they tell patients about the risks and benefits in these situations.
As with the prostate cancer meeting, the heterogeneity of perspectives was remarkable, but we discovered that most women were primarily interested in reducing the risk of recurrence regardless of treatment toxicity.
After hearing a description of the treatment schedules and toxicities for CA and CMF, more than half of the survivors indicated they would want to be treated with chemotherapy for a one percent improvement in survival. The greatest concern about CA was the potential cardiotoxicity, but alopecia was also an issue. Those preferring CA did so primarily because of the convenience in treatment scheduling compared to CMF. Patients also preferred anastrozole over tamoxifen, mainly because of concerns over endometrial cancer and thrombosis (Figure 2).
One of the most fascinating sidelights of these meetings was that in New York, the faculty presented qualitative estimates on the potential benefits of various interventions, but in Florida, the panel presented the absolute projected benefits for treatment based on Peter Ravdin's Adjuvant! computer model.
In a high-risk, node-positive situation, this dramatically changed how participants voted on endocrine therapy in a postmenopausal patient (Figure 3).

Designing credible continuing education programs about adjuvant treatment is a challenge because our physician audience knows that the available data is often inconclusive. To this end, they regularly seek the perspectives of research leaders whose professional lives revolve around breast cancer. However, the patient meetings offer “food for thought” about another perspective that must enter the equation. Our simple, highly unselected CME needs assessment experiment with breast cancer survivors reinforces the importance of offering available information to women who wish to receive it.
We have concluded that absolute reductions in risk of relapse and death from breast cancer must be part of the menu of information options presented. It is not acceptable to tell an inquisitive patient that therapy will reduce her chance of relapsing by, for example, 30 percent, when this number refers to relative risk reduction. For a woman whose absolute risk of relapse is, for example, 10 percent, she must be advised that treatment will not change her outcome more than 95 percent of the time.
Similarly, we also firmly believe that one cannot simply ignore the ATAC trial data and only discuss tamoxifen as an option for adjuvant endocrine therapy in postmenopausal women. According to the Ravdin model, a postmenopausal woman with an ER-positive tumor and a 10-year relapse risk of 60 percent without treatment will have that risk lowered to 45 percent with tamoxifen but to 38 percent with anastrozole. This, of course, assumes that the 47-month ATAC data will continue its current trend, but in the face of these numbers, patient preferences were clear-cut.
Breast cancer has set an example for the rest of oncology in terms of patient advocacy and education. Prostate cancer research leaders frequently cite the breast cancer clinical research experience when they look into the future.
Our foray into patients' meetings clearly revealed that one cannot generalize or assume how a person with cancer will react to challenging decisions, such as those related to adjuvant systemic therapy. These dilemmas will always demand personalized and time-consuming consultations.
—Neil Love, MD
|
