| You
are here: Home: BCU 6|2003: Kathy
S Albain, MD

Edited comments by Dr Albain
Phase II trial of gefitinib in women with metastatic
disease
Our study was an investigator-initiated, multicenter, Phase II
trial in women who had received any number of prior chemotherapy
regimens for metastatic disease; patients receiving first-line
therapy were also eligible. Patients who received multiple regimens
dominated the population, but there were a few who had no prior
chemotherapy. The trial was unique in that the women had to be
actively progressing on chemotherapy to qualify for the trial.
They also had to have available tumor specimens for the molecular
substudy.
Patients were treated with 500 mg/day of gefitinib and assessed
every eight weeks. The primary endpoint of the trial was the clinical
benefit rate. The design was similar to hormonal therapy trials
in which complete and partial responses or stable disease for six
months or more qualified as a clinical benefit.
Clinical benefit
One patient had a partial response, and two patients had stable
disease for more than six months. In addition, there were six more
patients with stable disease up to six months. The median progression-free
survival was 57 days, but we had patients whose disease was free
of progression for 205 or more days. In this group of women who
had been actively progressing on prior therapies, the median progression-free
survival was clustered at the first assessment point, but there
were a number of patients who stayed on gefitinib for several months
after that.
The patient whose disease had a partial response had received
high-dose chemotherapy and every possible chemotherapy drug that
is active in breast cancer. She was on gemcitabine for three cycles,
vinorelbine for a few cycles and kept progressing through each
of those. Then, her lung metastases and breast mass had a partial
response to gefitinib.
The trial was not designed to assess quality of life or pain,
but five out of 12 patients with bone pain had dramatic relief
of their pain. It didn’t matter that they had a few more
liver or lung metastases; they went off of the narcotics. Although
they were classified as having progressive disease, they didn’t
want to stop the gefitinib, because it ameliorated their pain.
I had two patients with bone pain who pleaded with me not to
stop their gefitinib; they wanted to stay on it because they hadn’t
felt so well in months. This observation from our trial obviously
needs to be followed up, but I am convinced that this is not a
placebo effect. These women had undergone every conceivable therapy,
and they didn’t suddenly just miraculously go off all of
their pain medications. Perhaps gefitinib will prove to be beneficial
for patients with bone metastases.
Side effects
There was no pulmonary toxicity in this trial. The acneform rash
and diarrhea were very similar to that found in patients receiving
gefitinib for lung cancer. The usual management for the side effects
included a short drug holiday for up to 14 days, which usually
helped. Four patients had a dose reduction from the 500 mg to 250
mg to ameliorate toxicity.
The rash was usually on the face or trunk, and it was typical
acne. Sometimes it was pruritic or painful. Other patients had
a rash that wasn’t classic. The usual skin care regimes worked
for the acneform rash. There was nothing unusual about the diarrhea,
and we managed it with the common treatments.
Future trials with gefitinib
Studies combining gefitinib with chemotherapy as primary neoadjuvant
therapy will be conducted. Other studies will likely be performed
in patients with lower-bulk disease — perhaps patients whose
disease has had a complete or partial response from primary therapy.
Those types of trials are worthwhile, as are those with other growth
factor pathway inhibitors that will try to maximize inhibition
of the cross talk among the epidermal growth factor receptor (EGFR)
family. Studies combining gefitinib with antiestrogens would also
be of interest.
Intergroup trial 0100
Intergroup trial 0100 enrolled postmenopausal women with node-positive
and ER-positive breast cancer. The trial stratified patients by
nodal status (1-3 versus 4 or more), progesterone-receptor status
(negative or positive) and time from surgery. The patients were
randomized to tamoxifen alone for five years, classic CAF followed
by five years of tamoxifen or CAF with concurrent tamoxifen starting
on day one.
The trial enrolled 1,477 eligible patients of which 32 percent
were 65 years of age and older, and 13 percent were 70 years of
age and older. The first objective was to combine the two CAF arms
and compare them to the tamoxifen-alone arm.
The eight-year, disease-free survival for tamoxifen alone was
55 percent and 67 percent for CAF followed by tamoxifen. That represented
an absolute difference of 12 percent, which is almost unheard of
in an adjuvant trial. Overall survival for tamoxifen alone and
CAF followed by tamoxifen was 67 percent and 73 percent, respectively.
The CAF with concurrent tamoxifen arm was in the middle.
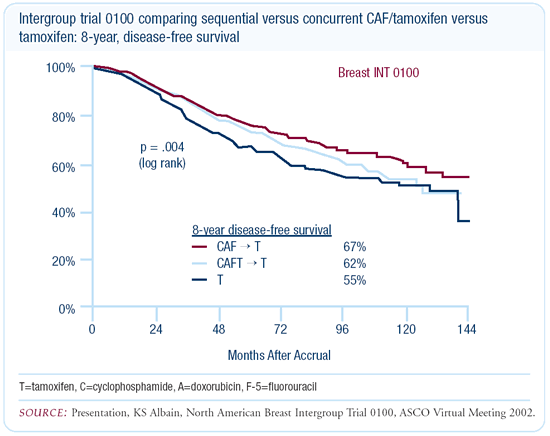
In an updated analysis presented at ASCO 2002, we reported for
the first time the breakout between the two chemotherapy arms.
We still reported a major benefit for the combined chemotherapy
arms compared to the tamoxifen-alone arm. That data was very mature
for both disease-free survival and overall survival.
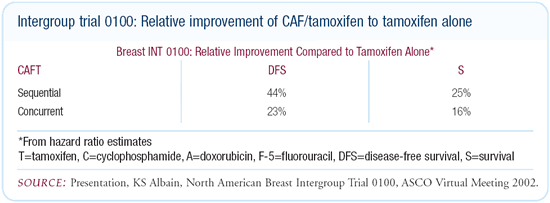
We found the eight-year, disease-free survival to be 76 percent
for the CAF with sequential tamoxifen arm, 62 percent for the CAF
with concurrent tamoxifen arm and 55 percent for the tamoxifen-alone
arm. Although there was still a benefit for chemotherapy compared
to tamoxifen alone, 50 percent of the chemotherapy benefit was
lost by giving it concurrently with tamoxifen. Frequently, I’m
asked whether anastrozole would have the same effect when combined
with chemotherapy, and there’s just no data for that. Would
shutting down estrogen production decrease the cycling of the cells,
and would that matter? Or is it strictly an effect caused by tamoxifen
interfering with drug uptake, and maybe anastrozole would not?
I don’t know the answer to those questions.
Nonprotocol management of women with HER2-positive,
metastatic breast cancer
In the Slamon pivotal-trial data, there was a survival benefit
in a group of very poor-prognosis patients who were given a trastuzumab/chemotherapy
combination compared to a taxane or anthracycline-containing regimen
alone — despite crossover to trastuzumab. Therefore, I’m
a proponent of giving a taxane with trastuzumab, and now I will
give a platinum agent also in hopes of optimizing survival.
There is more myelosuppression associated with trastuzumab/paclitaxel
plus carboplatin than trastuzumab/paclitaxel. I’ve also been
administering carboplatin/docetaxel with trastuzumab. With either
of those regimens, I now give one dose of pegfilgrastim on day
two in an effort to avoid the somewhat troublesome neutropenia.
Pegfilgrastim in clinical practice
Since I’ve been using more of the platinum/taxane combinations
in breast cancer, I like to prevent patients from being hospitalized
due to neutropenic fever. I’ll also use pegfilgrastim if
I’m administering an anthracycline and docetaxel in the neoadjuvant
setting or for rapid reduction in tumor burden. I’ve used
that regimen enough to know that the patients will have a problem,
therefore, I use pegfilgrastim with the first cycle.
Future directions of breast cancer clinical
research
The exciting era ahead will be to determine how to use the targeting
agents and how to use a patient’s own profile to determine
which of these targeting agents should be prescribed. The bigger
picture involves tapping into the microarray from an individual
patient to select an optimal adjuvant regimen.
Another very exciting area is the upcoming analysis of our Intergroup
data bank of very small tumors that were archived in the 1980s.
Now, we have all of these markers that we can analyze, so we can
come up with a prognostic score to determine who should receive
adjuvant therapy.
There are exciting areas for survivorship and special populations
research that I’m involved in through the committee I chair
in SWOG — the Committee on Women and Special Populations.
One such study we’re about to mount is an ovarian protection
study in women with ER/PR-negative disease who are ready to start
adjuvant chemotherapy. They will be given a short course of an
LHRH during their adjuvant therapy to put the ovaries into a rest
mode until they complete their chemotherapy.
Late cardiac effects of women randomized to
CAF versus CMF
Dr Patricia Ganz reported on the late cardiac effects in women
who were randomized to CAF or CMF. Five years after therapy, there
was no significant difference in the incidence of ejection fractions
that were below normal, but the mean ejection fraction was significantly
lower for the women randomized to CAF. After another five years
of follow-up, we’re going to repeat the analyses.

Select publications
|
