| You
are here: Home: Web
Guide 02- Surgeons: Gabriel
N Hortobagyi, MD

Edited comments by Dr Hortobagyi
Implications of the ATAC trial in clinical practice
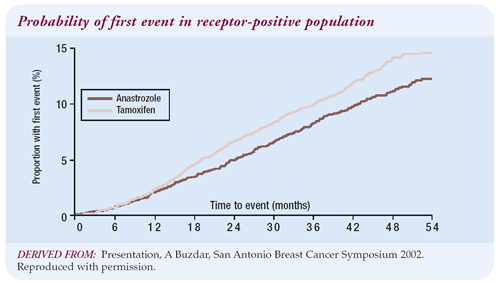
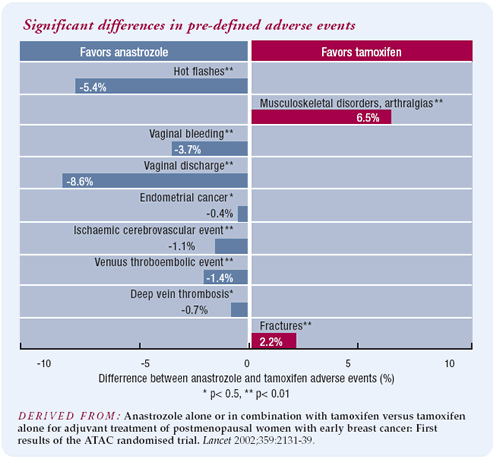
The results of the ATAC trial are quite compelling. Even if you
assume for the sake of argument that the curves will come together
with further follow-up, the safety profile of anastrozole is still
clearly better than tamoxifen. I cannot prevent endometrial cancer
short of removing the uterus, but I can prevent or treat osteoporosis
and fractures. Since the safety profile of anastrozole is better
than tamoxifen and it is therapeutically superior, I have a problem
not offering anastrozole to my patients — not as a neutral
choice but as a better choice. I do discuss with my patients the
enormous amount of clinical experience we have with tamoxifen, but
if my sister developed breast cancer today, I would certainly recommend
anastrozole as opposed to tamoxifen.
Use of other aromatase inhibitors in the adjuvant
setting
I do not use the other aromatase inhibitors in the adjuvant setting
because there are no data. While we have to extrapolate in a number
of situations, I do not see an advantage for the other aromatase
inhibitors from the existing data. It is possible that some time
in the future someone will show a distinct advantage of one of these
other agents, but at this point, the data were generated with anastrozole,
so I use anastrozole.
Recommending adjuvant anastrozole based on early
trial results
The ASCO technology assessment that does not support the use of
adjuvant anastrozole outside a clinical trial is based on fear of
the unknown in the face of the single largest clinical trial ever
conducted in the adjuvant setting. We have no comparable trial in
the history of medical oncology or breast cancer, and there is no
other tumor type with so many well-planned clinical trials conducted.
We are in a leadership position in oncology, and we can’t
advocate doing the best trials and then ignore the results of those
trials. Every single trial we do brings with it some of the unknown.
We started to move over to tamoxifen well before we had a five-year
followup. I remember when Michael Baum presented the early data
from the NATO trial in 1982. It had less than two years of follow-up,
and he was already publicly talking about the advantages of adjuvant
tamoxifen — and the NATO trial pales in size and design in
comparison to the ATAC trial. We have very compelling data about
anastrozole from the ATAC trial, in terms of its therapeutic and
safety profile superiority. I would be doing a disservice to my
patients who are candidates for adjuvant anti-aromatase therapy
by not presenting the data. I also present tamoxifen as an option,
but in the last six months about 60 percent of my postmenopausal
patients chose anastrozole rather than tamoxifen. There is no right
or wrong decision, but for me, there are compelling data to prefer
one versus the other.
Select publications
|
