| You
are here: Home: BCU 2|2003: Michael
Baum, ChM, FRCS

Edited comments by Dr Baum
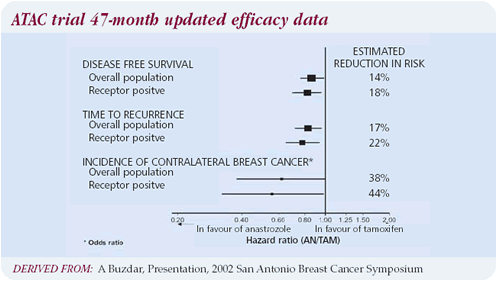
Updated data from the ATAC trial
The new ATAC trial data gives me comfort and a sense of vindication
that we waited a year before starting to make therapeutic recommendations.
Last year, I publicly supported the ASCO technology assessment.
Last year, I needed persuasion to use adjuvant anastrozole. It was
a nice option if tamoxifen could not be tolerated or was contraindicated.
This year, however, with the updated efficacy and safety data,
my position has changed. Now, my default therapy for estrogen receptor-positive
postmenopausal women is anastrozole unless contraindicated. We have
another year of follow-up in the ATAC trial, and I am impressed
by the separation of the curves. The safety update is also comforting.
The fracture rate isn’t racing away, the relative risks are
stable and the other safety profile issues strongly continue to
favor anastrozole.
Monitoring bone mineral density in women on anastrozole
Loss of bone mineral density with anastrozole can be monitored.
We don’t withhold chemotherapy because we’re worried
about white cell count — we give it, but we monitor the white
cell count. Osteopenia is not a dramatic crisis like neutropenia.
I would check bone mineral density at diagnosis, upon initiation
of anastrozole and annually thereafter. I would intervene with a
bisphosphonate if it started to fall. The one adverse effect favoring
tamoxifen over anastrozole can be managed.

Chemotherapy in the ATAC trial
Chemotherapy use is an unfortunate confounding variable in the
ATAC trial. Those women with the greatest lymph node involvement
and worst prognosis received chemotherapy. Although there are wide
confidence intervals, tamoxifen and anastrozole appear equivalent
in this subgroup of patients.
We argue about the reasons for this among members of the steering
committee. Aman Buzdar, for example, believes that this equivalence
could have resulted from the heterogeneity in chemotherapy regimens
used. I believe the most likely explanation is statistical noise,
because there were so few events in this subgroup of 20 percent
of the whole study population. The only other explanation I can
come up with is that the endocrine therapy was given after the completion
of chemotherapy, not synchronously. We now know that tamoxifen performs
better if delayed until after the completion of chemotherapy. This
is not a reason to withhold anastrozole in the subgroup of women
receiving chemotherapy. Even in these women, anastrozole is equivalent
to tamoxifen — equivalently good. Despite possible equivalence
in efficacy, the tolerability and safety profile favors anastrozole.
One important issue is the reduction in gynecological symptoms
with anastrozole. Aside from a decrease in endometrial cancer, there
is a 50 percent reduction in the incidence of vaginal bleeding,
a condition that is frightening and leads to overinvestigation and
excess hysterectomies. This reason alone is an argument to use anastrozole.
Other aromatase inhibitors in the adjuvant setting
Bill Miller and Per Lonning warn us not to make assumptions about
the efficacy and tolerability of the three aromatase inhibitors
because there are very subtle differences between them. We cannot
extrapolate from ATAC to exemestane because there may be differences
in efficacy and tolerability between the steroidal and nonsteroidal
agents. Exemestane is a permanent anti-aromatase with weak androgenic
effects.
Letrozole and anastrozole are nonsteroidal aromatase inhibitors,
but letrozole appears to produce a slightly greater reduction in
aromatase. While one might predict this would cause greater efficacy,
the tiny trickle of estrogen left by anastrozole may be important
for tolerability. We cannot assume a class effect — we must
do the trials.
Follow-up of patients enrolled in the ATAC trial
After a long debate, and at the recommendation of the data-monitoring
and safety committee, we agreed to close the combination arm of
ATAC. We are in the process of doing this, and although it sounds
simple, it isn’t. We are obtaining ethical approval and going
through the individual clinician investigators to unblind the patients
on the combination arm. We are providing leaflets and information
to help the clinicians and patients reach a decision about either
stopping both drugs or continuing on one. We are not making recommendations
about what should be done.
The combination arm results looked similar to tamoxifen last year,
but they are beginning to look worse. There is a relative risk of
1.1 favoring tamoxifen over the combination, which is not statistically
significant by any means, but it just might continue to diverge.
When the trial results became public last year, we were concerned
that many patients would want to be unblinded. This would have diluted
the study and resulted in a crossover. But, it has been a nonevent.
We re-consented women on the monotherapy arms of the trial, and
it hasn’t posed a problem. Only a handful of patients asked
to be unblinded.
We are not unblinding the monotherapy arms of the trial, because
most women in the tamoxifen arm have been on tamoxifen for four
years. We cannot recommend switching to anastrozole, because we
have no idea what might happen. The monotherapy arms will remain
blinded after completion of therapy to avoid bias in follow-up.
Cultural differences in interpretation of chemoprevention
data
There is a cultural clash with regard to chemoprevention. The
IBIS-II trial doesn’t please the Americans at all, because
it has a placebo arm. The study will be anastrozole against placebo,
because in the United Kingdom and Australia, we do not believe tamoxifen
is the standard of care. A substratum of IBIS-II will look at anastrozole
versus tamoxifen in DCIS, mirroring what Richard Margolese is doing
in the NSABP.
We thought that NSABP P-1 reported prematurely, and because there
has been some crossover, we will learn nothing more from this trial.
Jack Cusick, principal investigator of the IBIS trial, did a meta-analysis
of the four tamoxifen prevention trials, which showed a significant
prevention or delay of estrogen receptor-positive breast cancers,
but significant adverse events from tamoxifen. The net effect on
all-cause mortality is a relative risk of 1. We believe we have
proof of principle of tamoxifen prevention, but cannot recommend
tamoxifen, because thousands of women would be subject to the side
effects of tamoxifen, including thromboembolic disease and death
— to delay a few estrogen receptor-positive breast cancer
events. The net health improvement is zero.
Even if the results of IBIS-II play out, I'm not sure whether
anastrozole will be used for prevention. Years ago, when we embarked
on the tamoxifen prevention trial, I calculated that two percent
of postmenopausal women will develop breast cancer over a 10-year
period. Amagic drug that prevented half of breast cancers would
reduce this to one percent. We are exposing 99 out of 100 women
to side effects to benefit of one woman. Unless the drug has other
beneficial effects, you have a problem.
I predicted that chemoprevention in premenopausal women would
have to be a contraceptive that also prevented cancer, which is
not implausible. For postmenopausal woman, an ideal agent would
prevent osteoporosis as well as breast cancer — thus, raloxifene
is a good bet. The mistake with the STAR trial is the tamoxifen
arm rather than placebo control.


Anastrozole in the prevention setting
Some might argue that the reduction of contralateral breast cancers
in ATAC looks less promising with the updated data than with the
original data — it has gone from about a 60 percent to about
a 50 percent relative reduction in contralateral breast cancer in
the estrogen receptor-positive group. We had the same experience
early on with tamoxifen. The extremely dramatic difference seen
at three years was reduced over the next few years. This suggests
that these endocrine agents don’t prevent cancer, but rather
delay the appearance of cancer. Perhaps anastrozole delays the appearances
of breast cancer for longer than tamoxifen.
I am very confident that anastrozole will reduce the risk of estrogen
receptorpositive breast cancers — the adjuvant setting will
predict the preventive setting. The issue to me is the trade-off
and harm/benefit equation.
Trials of ovarian suppression plus aromatase
inhibitors in premenopausal women
Combining ovarian ablation with aromatase inhibitors in hormoneresponsive
premenopausal women is a very attractive proposition. We must take
our hats off to the Austrian collaborative group, which has emerged
as a world leader over the last few years. Its study design is extremely
elegant and rational. I will watch this beautiful study with great
interest.

Breast conservation rates in the ATAC trial
Gershon Locker presented breast conservation rates in the ATAC
trial at San Antonio. The dramatic finding is that breast conservation
is much less common in the United States than in the United Kingdom
and other countries. This study shows the beauty of this incredible
international database, which allows us to explore cultural differences.
It is fascinating that the two countries with the highest rates
of breast conservation were France — which is not unexpected
— and Brazil. Brazil is obsessed with the “body beautiful,”
but in addition, most Brazilian radiotherapists trained in France,
so we see an interesting cultural issue.
In fairness to the Americans, we should not overinterpret these
data. The United Kingdom is a small country in which everyone lives
within 100 miles of a radiotherapy center. In contrast, parts of
the United States are thousands of miles from a radiotherapy center.
Radiation therapy is six weeks of treatment. I can sympathize with
surgeons and women for whom it is just impractical to have breast-conserving
surgery.
Intraoperative radiation therapy
This technology, in theory, could allow us to give all radiotherapy
at the time of surgery with a portable machine in a community hospital.
We have a neatly packaged mobile electron generator, which delivers
x-rays at the tip of the probe. You can remove the tumor, apply
a spherical applicator to the tumor bed cavity, wrap the tumor bed
around this applicator and deliver radiotherapy to the index quadrant.
The whole process adds only half an hour to the operating time.
This technique gives the biological equivalent dose of 50 Grey
to the tumor bed. The geometry is better than conventional radiotherapy.
Traditional conformal radiotherapy conforms to an uncertain shape.
With this method, we conform the cavity to the radiotherapy source,
so I think we'll do better than with conventional external beam
radiation.
We did a Phase II study in 40 patients, and although I distrust
Phase II studies, it appears extremely safe with excellent cosmetic
results. Only one woman developed ulcerated skin, which ultimately
healed. In this series, over the maximum four or five years of follow-up,
we have not had a single local recurrence.
Trial of intraoperative radiation therapy
We have opened an exciting trial, randomizing patients to conventional
postoperative radiotherapy versus intraoperative radiotherapy. We
have a descriptive study nested within a pragmatic trial design.
The pragmatic aspect is that we take all comers, providing that
the tumor size relative to breast size allows us to fit an appropriate
size applicator. Beyond that, we are only excluding those with lobular
invasive cancer or extensive intraductal cancer. We've also allowed
a descriptive entry. In other words, you can predetermine to admit
only small, well-differentiated, node-negative tumors in older women.
We’re hoping to enroll 2,000 patients in the study, so we
need to spread our wings. There is enormous interest, and we have
started randomization. We have groups in Australia, North America
and Germany.
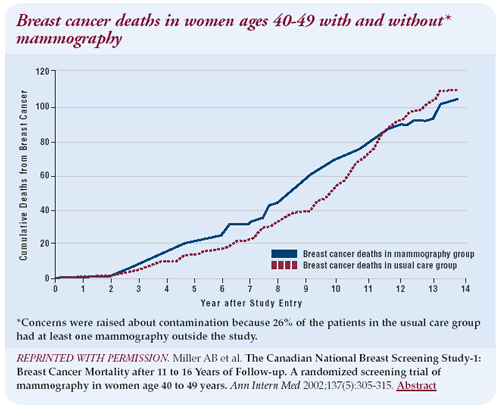
Mammography in women under age 50
The latest Canadian trial results published in Annals of Internal
Medicine in September do not demonstrate an advantage in breast
cancer mortality. In fact, there is an excess mortality from breast
cancer in women under 50 for the first 10 years of the study. This
excess mortality in the early years has been also been noticed in
the overviews of the screening trials as well.
I had a patient with screening-detected DCIS. After a biopsy, the
patient was advised to have surgery, however she chose not to have
treatment. She saw me six to nine months later with a breast full
of cancer. That is not the natural history of DCIS, but rather the
natural history of perturbed, incompletely excised DCIS. The biological
mechanism is perturbation of the tumor or its environment, which
induces angiogenesis.
Most in situ cancers are latent cancers, and angiogenesis is the
trigger from latency to invasion. Likewise, I believe most patients
with invasive cancer have metastases in dynamic equilibrium, which
progress and become life threatening when the system is perturbed
and angiogenesis is induced. Women with latent breast cancer or
occult metastases are living close to a chaos boundary, and we perturb
the system at our peril.
Informed consent for mammography in women over
50
My argument against screening women over 50 is not that it has
no effect, but that we are disingenuous in the way we invite women
to be screened. I passionately believe that women should make an
informed choice.
With systemic therapy, we bend over backwards to inform women
of the absolute benefits. We agonize whether a two or three percent
improvement in five-year survival is worth the “side effects,”
and we counsel our patients this way. We tell women that screening
will save their lives and reduce their risk of dying by 20 percent.
In absolute terms, we have to screen 1,000 women for 10 years to
save one life — one in a thousand. If we told women truthfully,
“If I screen you for 10 years, you will have one in a thousand
less chance of breast cancer death, but a significant risk of overdiagnosis,
false alarms, health insurance issues, unnecessary biopsies and
detection of duct carcinoma in situ, which never would have troubled
you,” many women would refuse it.
In the United States, I think there is a profit motive, and in
the United Kingdom, it's social engineering. I think it’s
almost fascistic to decide what is good for women and coerce them
to come forward for screening without telling them the whole truth.
Select publications
|
