|
You are here: Home: BCU 8| 2002: Dennis J. Slamon, MD, PhD
Edited comments by Dr Slamon
Initial phase I/II trastuzumab trials
In the first group of treated patients, we measured blood chemistries
and vital signs every few hours to look for any changes in critical
organ function. There was particular concern about the kidneys and
lungs. Yet, we did not see any toxicity other than a couple of patients
developing fevers from the drug infusion. Fever was treated with
acetaminophen and chills with diphenhydramine.
When we got to the highest dose levels, we saw a couple of responders.
The initial trials were conducted with a mouse monoclonal antibody.
We had to test the humanized form of the drug, and those phase I
studies were also done at UCLA.
The phase II trials confirmed what the animal models predicted.
Trastuzumab monotherapy, in refractory patients, appeared to have
a 12% to 15% response rate.
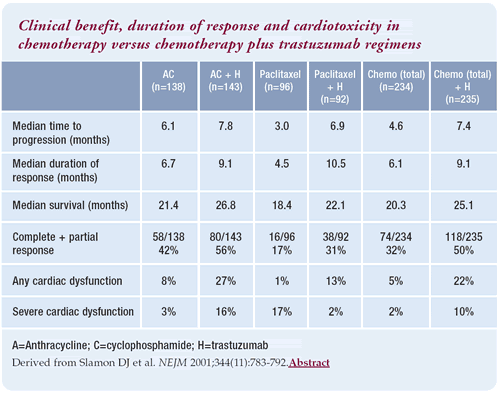
Pivotal phase III trastuzumab trial
We used a combination of an anthracycline and cyclophosphamide,
which was commonly used as first-line therapy in metastatic disease.
For those patients who had received adjuvant doxorubicin, paclitaxel
was utilized. Essentially, the patients were randomized to the best
available standard chemotherapy plus or minus trastuzumab.
In the phase III trial, the addition of trastuzumab led to a significant
improvement in response rate, response duration and time to progression,
respectively. A little-known fact from that trial is that the highest
response rate was seen in the anthracycline/cyclophosphamide/trastuzumab
arm compared to the paclitaxel/trastuzumab arm. Paclitaxel/trastuzumab
was ultimately included in the package label because of the toxicity
encountered with the other arm.
We were very encouraged with the improvement in the median time
to progression for the group receiving trastuzumab. Although it
is only a threemonth improvement, it translates, ultimately, into
a survival advantage. At four years of follow-up, trastuzumab decreases
the relative risk of death by 30% in women with truly HER2-positive
breast cancer — those who are FISH-positive.

Duration of response to trastuzumab
We have patients who are five and six years out from their initial
response to trastuzumab; some are still on it, and others are not.
Those still on trastuzumab were allowed, if they responded on the
trial, to continue. Many patients had very advanced disease so when
they had a complete response, some were reluctant to stop the trastuzumab.
There are also patients who have had complete responses and now
are off treatment. The longest living survivor treated with trastuzumab
was a patient in those phase I studies at UCLA. Initially, she had
16 nodules throughout her lungs and positive lymph nodes above the
clavicle. It is now about nine and a half years since she received
about four and a half months of trastuzumab.
Preclinical data on trastuzumab/chemotherapy
combinations
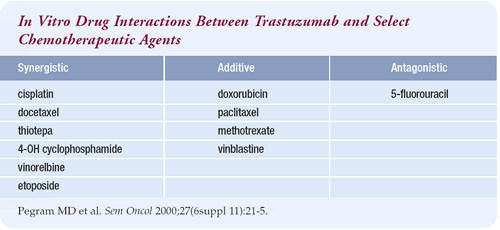
Preclinical studies demonstrated that trastuzumab in combination
with certain chemotherapeutic agents worked better than trastuzumab
alone. The drugs commonly used to treat breast cancer — doxorubicin,
paclitaxel, gemcitabine, methotrexate, vincristine and vinblastine
— tended to be additive with trastuzumab, and 5-FU was less
than additive.
The platinum salts — cisplatin and carboplatin — appeared
to be the most synergistic. After the platinum salts came docetaxel,
etoposide, vinorelbine and then the alkylating agents like cyclophosphamide.
We wondered why there was a difference between paclitaxel and docetaxel
since they both hit the same targets.
We learned that trastuzumab binds to the HER2 receptor and changes
signaling so that there is a transient decrease in DNA repair. The
platinum salts work by damaging DNA in a specific way, which is
reparable by DNA repair mechanisms that are shut down significantly
by trastuzumab. Docetaxel appears to be significantly superior to
paclitaxel in the ability to induce programmed cell death. When
trastuzumab and docetaxel are used together, the ability to induce
programmed cell death is increased significantly. We do not see
the same thing with paclitaxel.
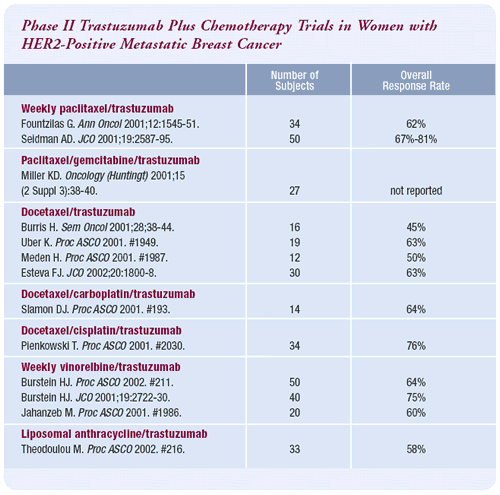
Phase II trials of trastuzumab/chemotherapy combinations
Trastuzumab plus chemotherapy — in particular the platinum
salts — gave us an objective response rate of 25%. Cisplatin
alone in refractory breast cancer is very inactive and not used
very often. It has a reported response rate of about 7%. But when
combined with trastuzumab, which has a response rate of about 12%,
there was more than an additive effect.
Cardiotoxicity with different chemotherapy combinations
When trastuzumab was used in combination with an anthracycline,
there was a significant increase in cardiotoxicity. In the pivotal
phase III trial, about half of the patients with cardiotoxicity
had class I and II, and the other half had class III and IV. Doxorubicin
alone is known to cause a 9% incidence of cardiotoxicity. Patients
with clinical cardiotoxicity can be treated with diuretics and ACE
inhibitors. When they improve, they can continue on trastuzumab.
Or, if the trastuzumab is discontinued, their cardiac function can
improve.
We believe the cardiotoxicity associated with paclitaxel/trastuzumab
was probably a recall phenomenon because of the data from Chuck
Vogel’s study in patients with HER2-positive disease who did
not receive chemotherapy. Those patients were treated with trastuzumab
alone, and the cardiac dysfunction rate was just under 4%. All were
subclinical. Trastuzumab by itself, in a population of patients
with minimal anthracycline exposure, was not a major cardiotoxin.
Next Page
Page 1 of 2
|
