|
You are here: Home: BCU 8| 2002: Dennis J. Slamon, MD, PhD
Biologic explanation for cardiotoxicity
Early studies looking at the distribution of HER2 receptors in
human tissues found many in the fetal heart but very few in the
adult heart. So we thought we would be okay in terms of the toxicity
profile.
However, subsequently in the adult heart of laboratory animals,
Ken Chien created a conditional knockout of the HER2 gene; he also
stressed the animals and found that they developed a dilated cardiomyopathy.
This proved mechanistically that the HER2 receptor plays some role
in maintaining cardiac performance and function. Therefore, if we
inhibit the HER2 receptor and stress the heart, we see this cardiac
problem.
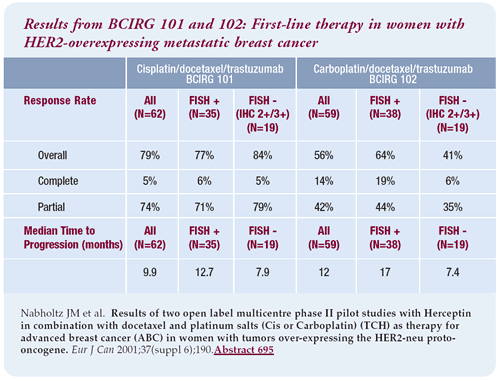
Carboplatin or cisplatin plus docetaxel/trastuzumab
(CTH)
The platinum salts in combination with docetaxel/trastuzumab have
a robust response rate. Two different phase II trials (BCIRG 101
and 102) have treated 124 patients. The response rate for CTH is
as good as that with an anthracycline/cyclophosphamide/trastuzumab
or paclitaxel/trastuzumab. For cisplatin/docetaxel/trastuzumab,
there is almost a five-month improvement in the median time to progression
for patients with FISHpositive tumors. For carboplatin/docetaxel/trastuzumab
the difference is even more dramatic.
This has been confirmed by Skip Burris in his study with carboplatin/
docetaxel/trastuzumab. Skip’s trial, the BCIRG trial and the
UCLA trial are all nonrandomized. The US Oncology trial being reported
at this year’s San Antonio meeting is showing the same thing
— the CTH combination is far superior to anthracycline regimens.
Preclinical data on trastuzumab/hormonal therapy
combinations
Clinical data was emerging indicating that patients with HER2-positive
breast cancer tended to be less responsive to tamoxifen. In fact,
that is exactly what the preclinical model demonstrated. Adding
trastuzumab significantly reverses tamoxifen resistance. This question
still needs to be addressed in clinical trials.
Fulvestrant works at the level of the activated receptor, binding
to the estrogen response element. Since fulvestrant does not allow
the activated receptor to do its job, it does not matter how the
estrogen receptor is activated. Fulvestrant might be effective in
HER2-positive breast cancer because it works lower down in the pathway.
Based on the available data, fulvestrant looks like the most promising
hormonal agent used in combination with trastuzumab. But the aromatase
inhibitors have not been tested as thoroughly as necessary.
Duration of trastuzumab therapy
There are no data currently addressing the optimal duration of
trastuzumab therapy. MD Anderson is trying to do a study of this,
but the pharmacokinetics of trastuzumab may intercede with the results.
After trastuzumab is discontinued, it is still on board when the
next chemotherapeutic agent is given. Because of the unique half-life
of the drug, these antibodies can be around for three to six months.
In the MD Anderson trial, one group will stop trastuzumab, and another
drug will be added, and the other group will continue the trastuzumab.
The group that stops the trastuzumab and has a second drug added
is still having that drug added in the presence of trastuzumab.
Based on preclinical data, our approach is to continue trastuzumab
after the patient has progressed on their first trastuzumab/chemotherapy
regimen and to add a different chemotherapeutic agent or I generally
use a second synergistic agent like vinorelbine. In the future,
we will probably be switching to a different biologic therapy. If
the patient is progressing rapidly on their second regimen of trastuzumab
and chemotherapy, my own approach is to stop the trastuzumab. If
the patient has a slow progression of their disease, I continue
the trastuzumab. It is a matter of clinical judgment in the absence
of clinical data.
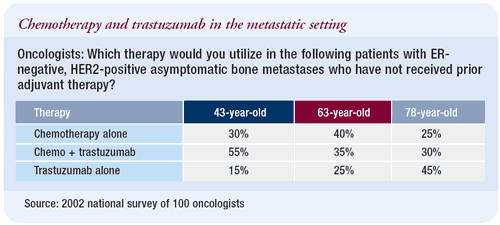
Patients with HER2-positive, ER-negative metastatic
disease
Systemic therapy is individualized to the patient. It depends
on what she has received previously, her general health, comorbid
diseases and a number of different factors. All things being equal
and the patient being capable, I opt for the most optimum interactive
combination of CTH. Trastuzumab can, however, be combined with vinorelbine,
capecitabine or gemcitabine.
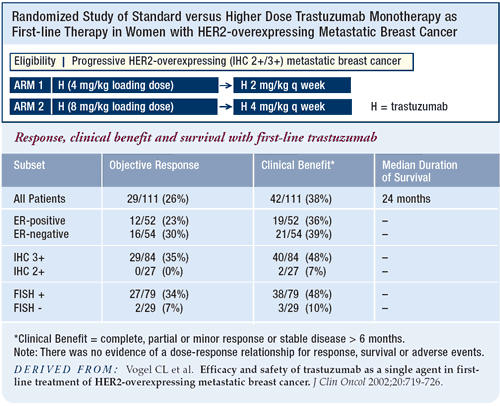
In terms of the response rate, trastuzumab monotherapy is inferior,
but the survival data looks comparable to that with the trastuzumab/chemotherapy
combination. Therefore, I am quite comfortable in a patient who
cannot tolerate or does not want chemotherapy to offer trastuzumab
monotherapy. It is not, however, my usual recommendation, which
is to exploit any potential synergies. HER2-positive breast cancer
is very aggressive, and we want to take our best shot at the disease.
Use of trastuzumab for metastatic disease
It is shocking to think that patients with HER2-positive metastatic
breast cancer would not be treated with trastuzumab up front. There
is a survival advantage with trastuzumab — the only agent
with a survival advantage in metastatic breast cancer. How can one
justify not using it?
Adjuvant trastuzumab trials
I felt strongly that trastuzumab should not have been combined
with anthracycline-based therapy when it moved into the adjuvant
setting. Yet, two U.S. cooperative groups have insisted on developing
this drug with anthracycline-based therapy.
In patients with HER2-positive metastatic breast cancer, which
is very aggressive and uniformly lethal with the old treatments,
taking risks makes sense as long as the patient and physician are
aware and patients are monitored. In the adjuvant setting, some
patients may be cured by the initial radiation and surgery.
Therefore, I think it is ill advised to put those HER2-positive
women on an anthracycline-based regimen — with the risk of
cardiac dysfunction — particularly if there are regimens that
look superior in terms of their efficacy based on preclinical synergy.
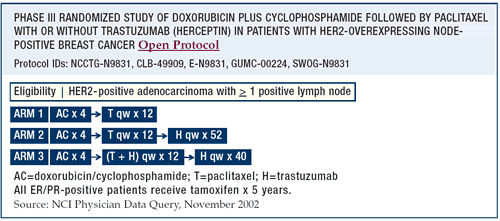
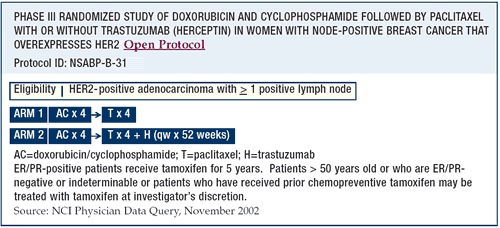
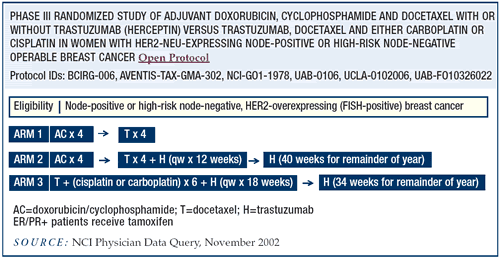
BCIRG-006
I wanted only two experimental arms — doxorubicin/cyclophosphamide
followed by docetaxel (AC -> T) and cisplatin or carboplatin
plus docetaxel/trastuzumab (CTH). But, there is a third arm with
doxorubicin and cyclophosphamide followed by docetaxel/trastuzumab,
which is very similar to the arms in the NSABP and the Intergroup
trials. We have not seen any cardiotoxicity signal yet.
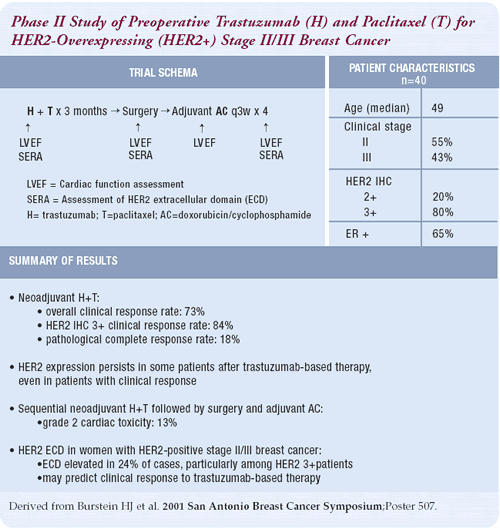
Neoadjuvant trastuzumab trials
Since we can obtain tissue, preoperative trials are a very good
strategy to learn about the biology of the disease. From those tissues,
we can hopefully learn which patients will be responders or nonresponders
and the degree of response. I am most interested in learning about
the downstream targets of the HER2 pathway and the intersecting
targets with the HER2 pathway.
Assessing HER2 status
Immunohistochemistry (IHC)
IHC was all we initially had available for testing, but early
on we saw that IHC was flawed. IHC has a false-negative rate of
about 18%. In a good laboratory, the false-positive rate for IHC
is probably a few percent; it goes up to 8% in general laboratories
and was as high as 40% in some of the early reported trials.
Mike Press has data demonstrating a 52% concordance with the Dako
HercepTest™ among Dako-approved pathologists. The College
of American Pathologists has done its own study, evaluating the
concordance between a central laboratory and pathologists in the
community. They are seeing similar trends.
Fluorescence in situ hybridization (FISH)
It has been consistently shown that FISH is superior to IHC. In
the Genentech data set, which has been looked at very critically,
the benefit with trastuzumab was not consistent in the patients
with IHC-positive disease. When those cases were analyzed, the bulk
of the benefit was seen in the patients with FISH-positive disease.
FISH is not a subjective test. If one can count dots, there should
not be falsepositives. If false-positive FISH results were a real
phenomenon, the College of American Pathology (CAP) study should
have detected it. CAP took cases it characterized and sent them
to pathologists in community practice, not university pathologists.
Ray Tubbs has done this study, and they are seeing great concordance.
Discordance between IHC and FISH
In two trials, the patients with IHC 3+ and FISH-negative disease
had a response rate of zero to trastuzumab-based therapy. In one
trial, two patients with IHC 3+ and FISH-negative disease responded;
those cases need to be reanalyzed to make sure they are indeed FISH-negative.
Since blocks were never kept, they had to use stained slides, take
the cover slips off, unstain the slides and then do the FISH test.
Patients with IHC 0 or 1+ and FISH-positive disease are HER2-positive.
By the traditional HercepTest™, those patients would have
never gotten trastuzumab. This problem with IHC is a function of
the fixation of the tumor when it goes into formalin.
Reason for false-negative IHC results
If we had frozen material on all patients at the time of diagnosis,
IHC would be just fine. The problem occurs because formalin works
by cross-linking proteins. HER2 is a protein that is progressively
cross-linked. The longer the tissue is in the formalin, the more
cross-linking and masking of the epitope occurs.
Cross-linking to other proteins covers up the HER2 epitope, which
is detected by the antibody. Dako has tried to introduce antigen
retrieval to make that better. Although one can put all kinds of
fancy scanners onto the tissue, if one does not control the fixation
of the tissue, there is no way one can control what is tested.
Benefit of FISH compared to IHC
If one wants to know whether a patient has the HER2 alteration,
one should do FISH testing. One should not do a default IHC and
only if they are 2+, then do a FISH. Using that algorithm, patients
without the HER2 alteration will be treated with trastuzumab, and
other patients with the HER2 alteration may not be treated. The
BCIRG trial we are conducting was designed with FISH as the only
criteria for assessing HER2 status.
Every patient should have their HER2 status assessed by FISH testing.
We do not use or recommend IHC. I think the day when just FISH testing
is used in the community is coming, and I hope it will be sooner,
rather than later.
A chronology of select breast cancer publications
by Dr Slamon
|
