| You
are here: Home: BCU 5|2001: Section 4
Section 4
Special Feature:Third Annual Lynn Sage Breast Cancer Symposium
BREAST CANCER
CLINICAL TRIALS: DETECTING MODEST BUT HUMANLY IMPORTANT ADVANCES
IN TREATMENT
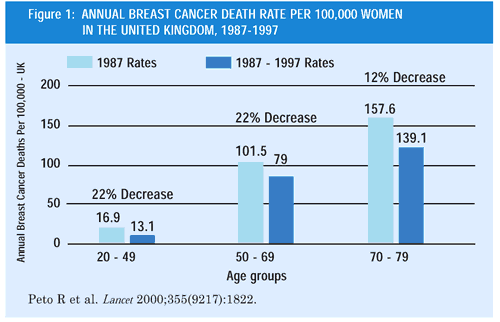
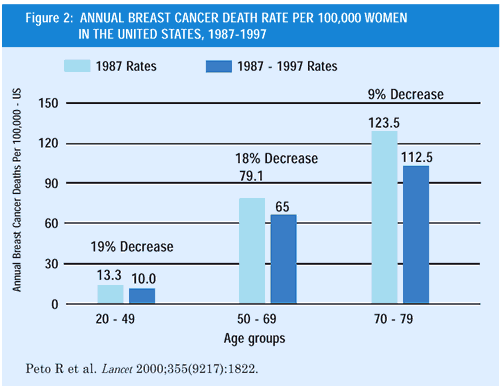
The recently reported decline in breast cancer mortality in the
United States and United Kingdom (Figures 1 and 2) has been attributed
to multiple factors, particularly the increased use of screening
mammography, adjuvant chemotherapy and endocrine therapy with tamoxifen.
Sir Richard Peto has estimated that in the United States alone,
10,000 fewer women a year are dying from breast cancer because of
these advances, and he has predicted that over the next decade mortality
rates will continue to decline, as established improvements in adjuvant
systemic therapy are implemented in clinical practice.
Peto first proposed the concept of large scale clinical trials
to detect modest but humanly important advances at the 1985 NIH
Consensus Conference on Early Breast Cancer. In an initial public
presentation of what was to become an ongoing series of key findings
from an international meta-analysis of randomized clinical trials,
the first Breast Cancer Overview revealed a sentinel groundbreaking
finding about adjuvant therapy that had a profound effect on the
structure of breast cancer clinical research.
Prior individual randomized studies had demonstrated that adjuvant
tamoxifen improved disease-free survival, but no statistically significant
impact on mortality was evident. Many observers concluded that adjuvant
systemic therapy — particularly “cytostatic” endocrine
therapy — would only delay recurrence without affecting the
long-term outcome.
Peto noted that there are two potential reasons that clinical trial
data fail to demonstrate an effect, such as mortality reduction:
1.The intervention has no effect on the outcome, e.g., adjuvant
tamoxifen does not reduce breast cancer mortality.
or
2.The intervention does have the effect, but the data are not
statistically powered to detect the effect, e.g., adjuvant tamoxifen
does reduce mortality, but there were not enough events —
in this case, deaths — observed in the individual trials
to detect that effect.
The statistical mantra for this concept is: “The lack of evidence
of an effect is not necessarily evidence of a lack of that effect.
”Specifically, researchers realized that one of the most salient
features of research planning was to focus on the expected number
of measured events, as opposed to just the size and follow-up of
trials.
In what could be viewed as a classic moment in breast cancer medicine,
Peto’s 1985 presentation demonstrated that combining the existing
randomized individual trial data on tamoxifen into a meta-analysis
revealed a clear-cut impact on mortality that was obscured by smaller
trials with relatively few deaths. Increasing recognition of the
importance of adequate statistical power in clinical trials has
led to the evolution of large cooperative clinical research groups
with the infrastructure to conduct studies with a sufficient number
of events to detect moderate but important improvements in outcome.
In adjuvant trials, recurrences and deaths are usually key measured
events, and for example, it became clear that for lower-risk study
populations, more patients and longer follow-up would be required
to observe an adequate number of these events. A fascinating footnote
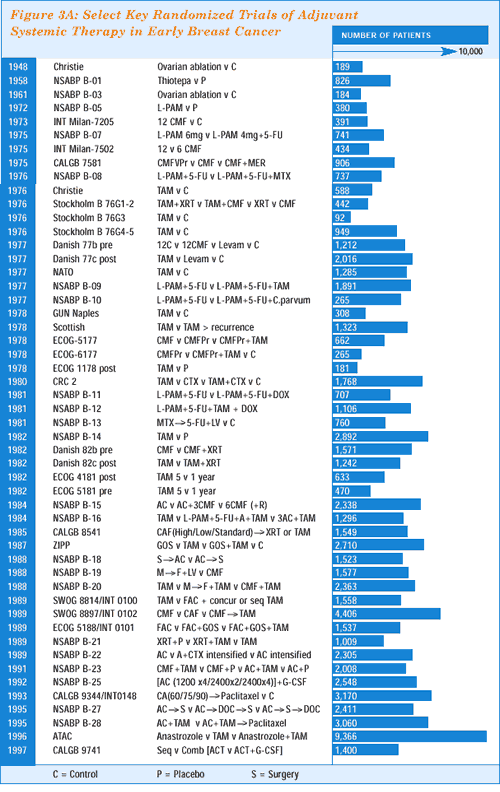
on this concept is the soon-to-be-reported ATAC adjuvant trial —
perhaps the largest individual randomized study ever conducted in
breast cancer — which has about ten times as many patients
as the largest trial in the original tamoxifen meta-analysis reported
by Peto in 1985.(Figures 3A,3B,3C)

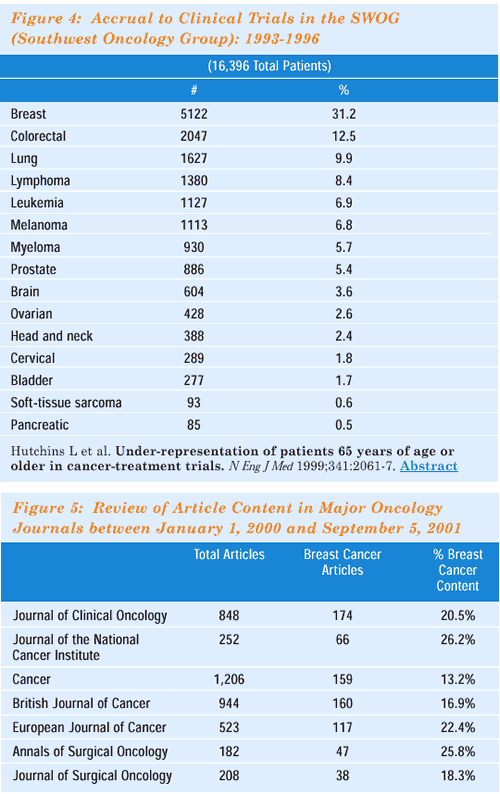
The importance of breast cancer in the spectrum of current clinical
research in oncology is demonstrated in Figure 4. This report from
one of the leading cancer cooperative research groups demonstrates
that about a third of patients entered in SWOG trials are women
with breast cancer, despite the fact that only about 15% of all
new cancer cases are people with this disease. Published reports
of clinical trials also reflect the importance of breast cancer
in oncologic medicine (Figure 5), and for example, the last 2000
San Antonio Breast Cancer Symposium included 437 scientific abstracts.
Physicians, nurses and other healthcare professionals caring for
breast cancer patients should understand the background, rationale
and design of these and other current randomized trials for several
reasons:
1.Current trial designs provide insight into how research leaders
interpret available research data on evolving treatment strategies.
The control arms of these studies are a commentary on what is considered
“standard,” and the experimental arms provide clues as
to what might be expected in the future.
2.Understanding the research questions being addressed prepares
clinicians for the next generation of clinical trial results.
The evolution of independent external data monitoring committees
sometimes leads to the seemingly precipitous dissemination of early
trial results, which can be challenging to implement into clinical
care unless there is prior familiarity with trial designs and objectives.
A good example was the sudden release in 1998 of initial data from
NSABP P-1, the “Tamoxifen Prevention Trial.” Clinicians
and researchers who were unfamiliar with the entry criteria and
objectives for this study suddenly had to integrate a somewhat complex
data set into clinical practice, but physicians who had participated
in this trial were already well-positioned to understand its clinical
implications.
3.All cancer patients deserve the opportunity to participate
in clinical research.
A disappointing three percent of breast cancer patients are enrolled
in clinical trials, although about half are interested in participation
when asked. As noted by SWOG, enrollment of elderly women is particularly
problematic.
Participation in clinical trials offers patients and physicians
many well-documented advantages, including assurance that treatment
and follow-up are state-of-the-art, and the gratification that comes
with helping move the field forward.
Peto’s estimate of 10,000 lives saved annually means that
every day, in the United States alone, about 25 women will be alive
and well, who would have died without the seemingly modest but humanly
important advances that have been made in diagnosis and treatment
of breast cancer in the last two decades.
A closer look at the core of this encouraging trend is the emergence
of large-scale randomized clinical trials that demonstrated the
benefits of screening mammography and adjuvant systemic therapy.
In essence, thousands of patients and healthcare professionals have
together woven a new set of lifesaving standards through clinical
research.
A decade or two from now,oncologists will likely be reading about
the results of the current trials listed in this book and hopefully
a new generation of patients will be alive and well as a result.
—Neil Love, MD
Page
1 of 6
Next page
|
