|
You
are here: Home: BCU 5|2001: Editor's Note
Editor
’s Note
LESSONS FROM
CLINICAL TRIALS ON LONG - TERM ADJUVANT ENDOCRINE THERAPY
Kathy Pritchard — a frequent guest on this series — often
raises issues in breast cancer research that no one else seems to
consider. It has been fascinating to observe the evolution of her
thinking over the years in interpreting trial results from a practical
clinical perspective. The last time we spoke, emerging data on third
generation aromatase inhibitors suggested greater antitumor efficacy
compared to tamoxifen. I was in the audience at the 1999 ECCO meeting
in Vienna when the first data emerged for anastrozole,and Kathy
was on her feet immediately, carefully querying the researchers
— who were overturning the classic breast cancer paradigm that
all endocrine therapies have the same efficacy.
Kathy prides herself on a conservative approach to applying research
data to patient care,and one year later,when most US oncologists
were routinely utilizing aromatase inhibitors as first-line therapy
for postmenopausal women with metastases,she was still “thinking
about it.” For our most recent interview — featured in
this issue of Breast Cancer Update — Kathy indicated that she
now is using AIs commonly as first-line therapy, but she also raised
a fascinating question related to an important issue being addressed
in a number of current randomized clinical trials.
While the aromatase inhibitors are rapidly moving into the
first-line treatment of metastatic disease,many of us are also involved
in adjuvant studies sequencing an aromatase inhibitor after two
to five years of tamoxifen. However, the ATAC adjuvant trial is
close to maturity and compares up-front Arimidex to tamoxifen to
the combination. The large studies of post-tamoxifen AIs may provide
interesting information that we may not use because we don’t
give the drugs that way anymore.I question whether these studies
of post-tamoxifen AIs are going to be relevant,because it may be
that the trials using aromatase inhibitors up-front as adjuvant
therapy will be the ones we ’re going to be interested in.
—Kathleen Pritchard, MD
Interviewing key breast cancer research figures for
this series has been an adventure, and Kathy’s provocative
but very salient comment reminded me of a series of interesting
related concepts investigators have discussed over the years on
the strategy of adjuvant endocrine therapy.
What has made this paradigm particularly interesting
is the huge body of laboratory research that clinical investigators
have turned to in seeking clues for trial strategies. At the center
of this has frequently been Craig Jordan, whose animal studies in
the ’70s and ’80s demonstrated the “cytostatic”
effect of tamoxifen and led to studies of long-term adjuvant therapy
in women with breast cancer.

A number of randomized trials and the International
Overview confirmed that increasing the duration of tamoxifen up
to five years was beneficial, but data on greater than five years
suggested that — if anything — there was a deleterious
effect of extending treatment.
As a result, in 1995, the NCI sent out an alert to
physicians recommending that outside of a clinical trial setting,
adjuvant tamoxifen should be given for five years.
National Cancer Institute clinical announcement:Adjuvant
therapy of breast cancer — tamoxifen update. Bethesda (MD):National
Institutes of Health;1995. Full-Text
The key NSABP trial was protocol B-14 in patients
with ER+,node- negative tumors.A recent paper updated this experience.
“Through seven years after reassignment of tamoxifen-treated
patients to either placebo or continued tamoxifen therapy,a slight
advantage was observed in patients who discontinued tamoxifen relative
to those who continued to receive it:DFS =82% versus 78% (P =.03),
RFS =94% versus 92% (P =.13), and survival =94% versus 91% (P =.07),
respectively.The lack of benefit from additional tamoxifen therapy
was independent of age or other characteristics.”
Fisher B et al.Five versus more than five years of tamoxifen
for lymph node-negative breast cancer:Updated findings from the
National Surgical Adjuvant Breast and Bowel Project B-14 randomized
trial. J Natl Cancer Inst 2001;93(9):684-90.Abstract
Interestingly, at about the same time that clinical trials were
demonstrating that extending tamoxifen beyond five years was not
beneficial, Craig Jordan was finding that cell lines exposed to
tamoxifen for prolonged periods eventually became dependent on tamoxifen
for growth. Subsequent research revealed an even more fascinating
finding — namely, that tamoxifen-dependent cells were actually
destroyed by exposure to physiologic levels of estrogen.
We took tamoxifen-stimulated tumors and retransplanted them
into animals for five years.Without treatment, the tumors grew sluggishly.
We then exposed these animals to either tamoxifen or physiological
levels of estrogen as you would see in a normal woman. Tamoxifen
stimulated the growth of the cells, but physiological levels of
estrogen caused the tumors to melt away. I’ve never seen anything
like this! This could explain how long-term tamoxifen works as an
adjuvant therapy. Upon stopping after about five years, the woman’s
own estrogen now comes back and seeks out all of these supersensitized
micrometastatic breast cancer cells and initiates an apoptotic mechanism.
The woman’s own estrogen consolidates the beneficial
effects of tamoxifen by causing a second antitumor action for months
after the tamoxifen has stopped. So we have developed a five-year
cycle within our model system where estrogen seems to attack the
supersensitized tumors that have been exposed to five years of tamoxifen,
creating apoptosis in breast cancer cells.
Another interesting laboratory observation is that if you
continue treating these animals with estrogen after five years of
tamoxifen, some of the tumors recur and start to grow again. If
you then take these now estrogen-stimulated tumors and transplant
them into a new generation, the tamoxifen now works as an antiestrogen
again.
—Craig Jordan, PhD DSc
This increasingly accepted practice of limiting adjuvant
tamoxifen therapy to five years was reinforced in the 2000 NIH Consensus
Conference,but during that same meeting,I interviewed Sir Richard
Peto, who told me that the existing data on tamoxifen duration was
inadequate to determine whether extending treatment beyond five
years would provide additional benefit.
We have really good evidence that five years of tamoxifen
is better than two years of tamoxifen. It’s not a big difference,
but it’s real. We don’t have comparable data in terms
of whether ten years is better than five years.A few thousand women
have been randomized, but most of them have been randomized so recently
that we have minimal follow-up. In terms of recurrences, we’ve
got a few hundred recurrences, whereas in the trials of five years
versus two years, we’ve got a few thousand recurrences. So,
we don’t have reliable information on whether ten years is
better than five years. And even worse, the recurrences that have
been observed are nearly all in the first few years after randomization,
where the carry-over benefit from that first five years of tamoxifen
is still going to be providing a lot of protection in the control
group.
Breast cancer is a disease with at least a 20-year natural history,
and the question is, “Would 10 years of tamoxifen be better
than five years of tamoxifen in providing protection against recurrence
in the second decade after diagnosis?” For a woman in her 50s
or 60s, 20-year outcome is very relevant. There are almost as many
breast cancer deaths in the second decade after diagnosis as in
the first decade after diagnosis, so we’ve really got to think
on a long-term scale. When you do that,then the idea that ten years
might be better than five years actually becomes quite interesting.
—Richard Peto, FRS
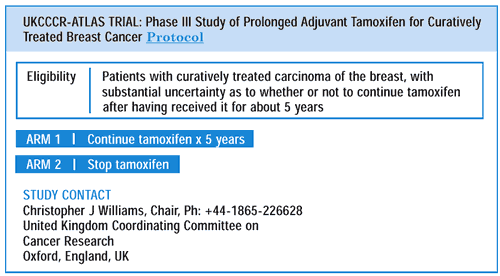
Peto and his colleague, Christina Davies, are coordinating the
massive Adjuvant Tamoxifen Longer Against Shorter (ATLAS) trial
to attempt to address this question by recruiting 20,000 patients
internationally, who are willing to be randomized to either continue
tamoxifen or stop five or more years after initiating therapy. A
similar study is the Adjuvant Tamoxifen Treatment, Offer More? (aTTom)
trial, in which patients also are randomly assigned either to discontinue
the drug or to receive tamoxifen for at least five more years. It
is not likely that the major findings from these trials will be
available for about 10 years. However, when one considers Kathy
Pritchard’s comment, this question of tamoxifen duration may
in the long run become a moot point if trials like ATAC demonstrate
that the initial adjuvant therapy should include an aromatase inhibitor.
The ebb and flow of breast cancer clinical research is very evident
in the issue of the optimal strategy of long-term endocrine therapy,
but there are other important lessons here that relate to all of
cancer research.
One key factor is the value of the translational model. Craig Jordan
and many other basic researchers have worked closely with the cooperative
breast cancer clinical trials groups, participating in a two-way
partnership that has resulted in randomized trials that have tested
key basic paradigms and provided tissue and serum samples leading
to new investigation in the laboratory.
Endocrine therapy has been viewed as the first targeted breast cancer
treatment that fully capitalized on the translational model, but
many others have followed, including trastuzumab and other agents
targeting EGFR, and tissue microarray studies evaluating the impact
of cytotoxic treatments.
Another important research concept that is evident in the example
of long-term endocrine therapy is the need for randomized trials
with sufficient statistical power to identify modest improvements
in outcome. Richard Peto has carried the torch for this issue and
over the last 15 years has taught us to critically evaluate findings
like the five-versus ten-year tamoxifen data.
The result has been the implementation of massive trials like ATAC
— which has more than ten times as many accrued patients as
the largest original adjuvant systemic studies reported in the late
’70s and ’80s.
To understand the clinical implications of this research database,
the Breast Cancer Update series has had as its focal point interviews
with key breast cancer laboratory and clinical investigators. We
have also focused a great deal of attention over the years on education
about the background, rationale and design of current randomized
clinical trials. Recently, our team put together an educational
exhibit and book on this topic for the Third Annual Lynn Sage Breast
Cancer Symposium, sponsored by Northwestern University. Bill Gradishar,
the meeting chair, and co-chairs, Monica Morrow and Craig Jordan,
frequent guests on our series, assisted in developing this “clinician-friendly”
first attempt at summarizing the key issues and questions being
asked by cooperative research groups.
The entire clinical trials book and exhibit are posted on BreastCancerUpdate.com,along
with hundreds of relevant links to journal articles, protocols and
cooperative group sites. The special feature in this issue of Breast
Cancer Update includes the first chapter of the educational supplement,
which lists major cooperative groups and their current phase III
randomized breast cancer clinical trials.
The rapid evolution of useful knowledge on breast cancer treatment
directly reflects the efficacy of these trial mechanisms in identifying
important questions and obtaining salient answers. Putting aside
Kathy Pritchard’s thought about the possible obsolescence of
post-tamoxifen aromatase inhibitor studies, it is interesting to
consider that it was only 15 years ago that trials clarified that
tamoxifen significantly made an impact on mortality in the adjuvant
setting, and third-generation aromatase inhibitors have only been
available to clinicians for about five years.
Because of an integrated and surprisingly efficient cooperative
clinical trials system, we now are exploring issues that are likely
to pay dividends to patients much more rapidly than anyone would
have imagined a decade ago. Even practicing clinicians who are not
accruing patients on trials will benefit from being informed about
where we’ve been, where we are and where we’re headed
in this fascinating journey.
—Neil Love, MD
Select Publications
|
|
