
 |

Select Excerpts from the Discussion
Management of patients with HER2-positive metastatic disease
Track 2
![]() DR LOVE: Antonio, how do you make a decision in terms of which
anti-HER2 therapy to administer to a patient with cancer relapse after
adjuvant trastuzumab?
DR LOVE: Antonio, how do you make a decision in terms of which
anti-HER2 therapy to administer to a patient with cancer relapse after
adjuvant trastuzumab?
![]() DR WOLFF: I’m surprised that, thus far, I haven’t had any patients who have
had cancer relapse after receiving adjuvant trastuzumab, and we were active
participants in NCCTG-N9831. Maybe the window of relapse will be early,
and it may plateau later on. Perhaps some of these patients who have not had a
relapse will not have one — and that would be wonderful.
DR WOLFF: I’m surprised that, thus far, I haven’t had any patients who have
had cancer relapse after receiving adjuvant trastuzumab, and we were active
participants in NCCTG-N9831. Maybe the window of relapse will be early,
and it may plateau later on. Perhaps some of these patients who have not had a
relapse will not have one — and that would be wonderful.
The major concern is that we have absolutely no idea of what prior trastuzumab will mean in this situation. I am not sure what these artificial boundaries of six, 12 or 24 months mean, but at some point you need to draw the line.
I believe that if someone has an immediate relapse within the first year after trastuzumab, I would be more nervous about attempting to use a trastuzumab-containing regimen and may proceed to lapatinib. But again, I believe we are making artificial decisions.
![]() DR GRALOW: You can select one or two years. If it’s a short interval since the
completion of adjuvant trastuzumab, I believe lapatinib plays a role, although
I might go back to trastuzumab at some later time. If it’s a longer interval, our
group is comfortable with trastuzumab.
DR GRALOW: You can select one or two years. If it’s a short interval since the
completion of adjuvant trastuzumab, I believe lapatinib plays a role, although
I might go back to trastuzumab at some later time. If it’s a longer interval, our
group is comfortable with trastuzumab.
Integrating bevacizumab into clinical trials and practice
Track 13
![]() DR LOVE: Cliff, can you discuss the planned CALGB trial evaluating
first-line therapy with bevacizumab in combination with paclitaxel, nab paclitaxel or ixabepilone (2.1)?
DR LOVE: Cliff, can you discuss the planned CALGB trial evaluating
first-line therapy with bevacizumab in combination with paclitaxel, nab paclitaxel or ixabepilone (2.1)?
![]() DR HUDIS: Hope Rugo is a principal investigator of this prospective,
randomized, Phase III trial along with Alvaro Moreno from the NCCTG.
They proposed a Phase II randomized trial of ixabepilone versus paclitaxel
in combination with bevacizumab. We proposed a Phase III trial of weekly paclitaxel versus nab paclitaxel. CTEP asked us to collaborate and join these
two studies.
DR HUDIS: Hope Rugo is a principal investigator of this prospective,
randomized, Phase III trial along with Alvaro Moreno from the NCCTG.
They proposed a Phase II randomized trial of ixabepilone versus paclitaxel
in combination with bevacizumab. We proposed a Phase III trial of weekly paclitaxel versus nab paclitaxel. CTEP asked us to collaborate and join these
two studies.

Weekly paclitaxel, for three weeks out of four, with bevacizumab is the control regimen. The two experimental arms administer weekly ixabepilone or weekly nab paclitaxel, both with bevacizumab.
Significant correlative science studies are also built into the study. The cooperative groups wrote the study with progression-free survival as an endpoint. However, FDA-related discussions are ongoing as to whether or not this study will be adequate for drug approval and, therefore, how we should move forward.
Track 19
![]() DR LOVE: Chuck, what’s your opinion about the use of overall survival as
an endpoint in trials of metastatic breast cancer?
DR LOVE: Chuck, what’s your opinion about the use of overall survival as
an endpoint in trials of metastatic breast cancer?
![]() DR GEYER: It’s a problem to make overall survival your ultimate bar for a
disease in which we have so many therapies that have an impact on patients.
They maintain their performance status, and their volume of disease is
controlled by changing drugs around.
DR GEYER: It’s a problem to make overall survival your ultimate bar for a
disease in which we have so many therapies that have an impact on patients.
They maintain their performance status, and their volume of disease is
controlled by changing drugs around.
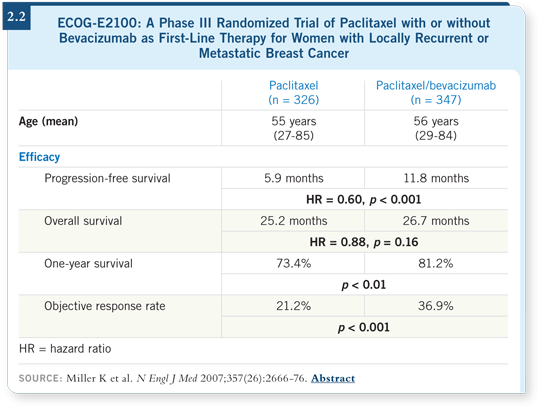
What bothers me about the ECOG-E2100 bevacizumab data is that a doubling of progression-free survival is substantial in breast cancer. We’re not talking about 1.5 to three months. In breast cancer, we’re talking about substantial time (Miller 2007; [2.2]).
![]() DR LOVE: Bob, what discussions have occurred within the NCCN Breast
Cancer Committee regarding the use of bevacizumab in metastatic disease?
DR LOVE: Bob, what discussions have occurred within the NCCN Breast
Cancer Committee regarding the use of bevacizumab in metastatic disease?
![]() DR CARLSON: Currently the use of bevacizumab and paclitaxel in the first-line
treatment of metastatic disease is included in the NCCN guidelines as an
option. The NCCN panel does not require FDA approval of an agent and a
specific indication to incorporate it into the guidelines, nor does FDA approval
mean that it is incorporated into the guidelines.
DR CARLSON: Currently the use of bevacizumab and paclitaxel in the first-line
treatment of metastatic disease is included in the NCCN guidelines as an
option. The NCCN panel does not require FDA approval of an agent and a
specific indication to incorporate it into the guidelines, nor does FDA approval
mean that it is incorporated into the guidelines.
![]() DR LOVE: Within the NCCN Breast Cancer Committee, how much
weight do you give to overall survival versus progression-free survival in the
metastatic setting?
DR LOVE: Within the NCCN Breast Cancer Committee, how much
weight do you give to overall survival versus progression-free survival in the
metastatic setting?
![]() DR CARLSON: The panel to date doesn’t focus specifically on progression-free
survival, overall survival or toxicity. It’s more a gestalt or a balance among the
experts on the panel in terms of weighing the different advantages.
DR CARLSON: The panel to date doesn’t focus specifically on progression-free
survival, overall survival or toxicity. It’s more a gestalt or a balance among the
experts on the panel in terms of weighing the different advantages.
One of the issues in this discussion, though, is that none of the results from ECOG-E2100 indicate that bevacizumab does not provide a survival advantage. They show that the survival differences are not statistically significant as they were analyzed. The p-value was 0.16, which means there’s an 84 percent chance that it is better (Miller 2007; [2.2]).
If a Wilcoxon analysis were performed on those data, it would become statistically significant. So some of this relates to your statistician and which method of analyzing the data you prefer.
EDITOR
Neil Love, MD
TOPICS
Treatment of Early Breast Cancer
- Select publications
Treatment of Metastatic Breast Cancer
- Select publications
Breast Cancer Update - Think Tank:
A CME Audio Series and Activity