
 |
||||||||||||||

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 5-6
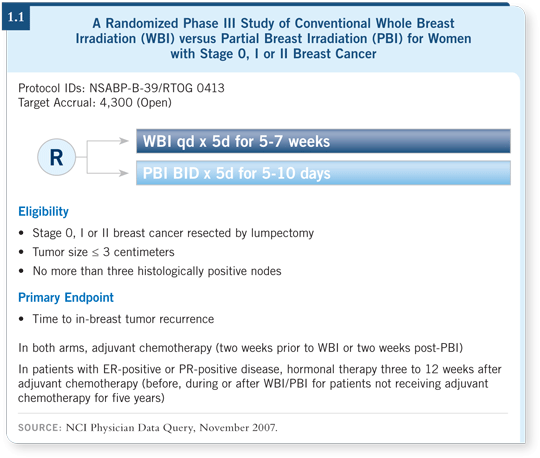
![]() DR LOVE: Would you discuss the NSABP-B-39 (1.1) trial and where we
are right now in terms of partial breast irradiation?
DR LOVE: Would you discuss the NSABP-B-39 (1.1) trial and where we
are right now in terms of partial breast irradiation?
![]() DR WHITWORTH: The most interesting aspect of B-39 is that it is answering
the questions that those of us who are using partial breast irradiation are
asking: Can it be used for patients with positive nodes? Can it be used for
patients at high risk — younger patients or patients with lobular tumors?
DR WHITWORTH: The most interesting aspect of B-39 is that it is answering
the questions that those of us who are using partial breast irradiation are
asking: Can it be used for patients with positive nodes? Can it be used for
patients at high risk — younger patients or patients with lobular tumors?
![]() DR LOVE: What’s your preferred method of PBI?
DR LOVE: What’s your preferred method of PBI?
![]() DR WHITWORTH: It depends on the patient. Some patients require the multi-catheter approach, whereas others are better off with a balloon and still others
fare better with 3D conformal.
DR WHITWORTH: It depends on the patient. Some patients require the multi-catheter approach, whereas others are better off with a balloon and still others
fare better with 3D conformal.
I prefer the balloon approach because it irradiates less breast tissue than the 3D conformal. Studies evaluating how much tissue volume actually receives the target dose demonstrate superiority for the balloon approach, mainly because the apparatus is held in a fixed place.
Breathing has no effect, so the target dose is delivered more uniformly even than in some of the multicatheter analyses (Weed 2005).
![]() DR LOVE: If PBI turns out to be an acceptable approach, how much of an
advantage will it offer women?
DR LOVE: If PBI turns out to be an acceptable approach, how much of an
advantage will it offer women?
![]() DR WHITWORTH: It’s a big advantage. We were shocked to find that approximately
15 percent of women do not receive radiation therapy because they live
too far from the facility (Athas 2000). The farther they live away from the
facility, the more likely they are to not receive it.
DR WHITWORTH: It’s a big advantage. We were shocked to find that approximately
15 percent of women do not receive radiation therapy because they live
too far from the facility (Athas 2000). The farther they live away from the
facility, the more likely they are to not receive it.
Thus the five-day cycle with the balloon is helpful to a lot of women. The other aspect that makes it more practical for women who live far away from the facility is that the American Cancer Society has arranged a place where people can stay for a few days while receiving treatment.
Tracks 10-11
![]() DR LOVE: How do you see the Oncotype DX assay fitting into clinical
practice now and evolving for the future?
DR LOVE: How do you see the Oncotype DX assay fitting into clinical
practice now and evolving for the future?
![]() DR WHITWORTH: This assay is one of the most exciting developments in breast
cancer in a long time. Surgeons and oncologists now have a way of deciding
which patients will benefit from systemic adjuvant chemotherapy. Historically,
we’ve all been frustrated because in some situations, out of 100 women, perhaps
six will have their lives saved by adjuvant chemotherapy. As a result, we have
treated a number of women who didn’t need it — we just couldn’t determine
who did and who didn’t. This genomic assay is now allowing us to identify
those groups of women.
DR WHITWORTH: This assay is one of the most exciting developments in breast
cancer in a long time. Surgeons and oncologists now have a way of deciding
which patients will benefit from systemic adjuvant chemotherapy. Historically,
we’ve all been frustrated because in some situations, out of 100 women, perhaps
six will have their lives saved by adjuvant chemotherapy. As a result, we have
treated a number of women who didn’t need it — we just couldn’t determine
who did and who didn’t. This genomic assay is now allowing us to identify
those groups of women.
![]() DR LOVE: The assay also has the potential to identify patients to whom oncologists
possibly wouldn’t have administered chemotherapy based solely on small
tumor size.
DR LOVE: The assay also has the potential to identify patients to whom oncologists
possibly wouldn’t have administered chemotherapy based solely on small
tumor size.
![]() DR WHITWORTH: Exactly. In fact, identifying this kind of patient keeps us
going. It’s like putting on glasses that allow you to see something you couldn’t
see before. You see not only the patients who don’t need it but also the
patients who by conventional criteria wouldn’t be treated and you’d miss.
DR WHITWORTH: Exactly. In fact, identifying this kind of patient keeps us
going. It’s like putting on glasses that allow you to see something you couldn’t
see before. You see not only the patients who don’t need it but also the
patients who by conventional criteria wouldn’t be treated and you’d miss.
Track 12
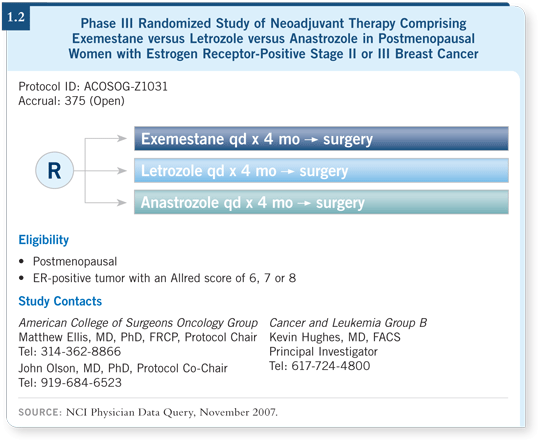
![]() DR LOVE: Could you review the ACOSOG study (1.2) on neoadjuvant
aromatase inhibitors?
DR LOVE: Could you review the ACOSOG study (1.2) on neoadjuvant
aromatase inhibitors?
![]() DR WHITWORTH: It’s critical for surgeons to be involved in neoadjuvant trials
in which you can obtain a biopsy of the lesion prior to some intervention
— whether it’s an aromatase inhibitor or something else — and then excise
the tumor and see what the biological effects have been. It’s also of practical
importance because now we’re identifying patients in whom we believe
hormonal neoadjuvant therapy will be much more appropriate than neoadjuvant
chemotherapy. Generally, those are postmenopausal women with ER-positive
disease.
DR WHITWORTH: It’s critical for surgeons to be involved in neoadjuvant trials
in which you can obtain a biopsy of the lesion prior to some intervention
— whether it’s an aromatase inhibitor or something else — and then excise
the tumor and see what the biological effects have been. It’s also of practical
importance because now we’re identifying patients in whom we believe
hormonal neoadjuvant therapy will be much more appropriate than neoadjuvant
chemotherapy. Generally, those are postmenopausal women with ER-positive
disease.
If you look at the information from the 21-gene (Oncotype DX) assay studies, patients who have a low likelihood of a great response to neoadjuvant chemotherapy seem to be the patients who have a better likelihood of benefit from hormonal therapy. So we are expecting to improve the breast conservation rate with neoadjuvant hormonal therapy in that series. That series will set up a much larger study to compare neoadjuvant hormonal therapy to neoadjuvant chemotherapy in the future.
![]() DR LOVE: Have you enrolled patients on this study?
DR LOVE: Have you enrolled patients on this study?
![]() DR WHITWORTH: Yes, and we have seen good responses. Furthermore, the
patients who have received neoadjuvant hormonal therapy have been happy with it. It’s statistically unlikely that metastasis could happen in this short time,
and most patients respond. We do expect to see confirmation of higher rates of
breast conservation.
DR WHITWORTH: Yes, and we have seen good responses. Furthermore, the
patients who have received neoadjuvant hormonal therapy have been happy with it. It’s statistically unlikely that metastasis could happen in this short time,
and most patients respond. We do expect to see confirmation of higher rates of
breast conservation.
| Table of Contents | Top of Page |