
 |
||||||||||||||

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1, 5

![]() DR LOVE: Can you discuss the conversation you had with this patient
regarding the findings from her biopsy?
DR LOVE: Can you discuss the conversation you had with this patient
regarding the findings from her biopsy?
![]() DR HYAMS: The final pathology demonstrated that the tumor was Grade II.
Although the ER status was 100 percent, the PR status was somewhat less
positive at 50 percent, which means the biological behavior of the tumor could
display some variability.
DR HYAMS: The final pathology demonstrated that the tumor was Grade II.
Although the ER status was 100 percent, the PR status was somewhat less
positive at 50 percent, which means the biological behavior of the tumor could
display some variability.
According to a study of patients from the original NSABP-B-14 trial, the concordance rate among pathologists grading Stage III and IV tumors was a little less than 50 percent when available slides from all of the patients treated with tamoxifen were sent to three different breast specialty pathologists (Paik 2004).
This suggests variability in tumor grading. Two out of three pathologists have some tendency to blur the lines between the Grade I and Grade II tumors and between the Grade II and Grade III tumors.
This patient’s tumor was considered a Grade II lesion, but we thought it might be useful to determine the likelihood of distant recurrence and suggested she consider participating in the TAILORx study. It would provide her with an opportunity to learn her recurrence score using the Oncotype DX assay and participate in a trial that would help us better identify which patients benefit most from chemotherapy with hormonal therapy or from hormonal therapy alone in the intermediate-risk group.
Track 8
![]() DR LOVE: After this patient enrolled in TAILORx, what did her
Oncotype DX results show?
DR LOVE: After this patient enrolled in TAILORx, what did her
Oncotype DX results show?
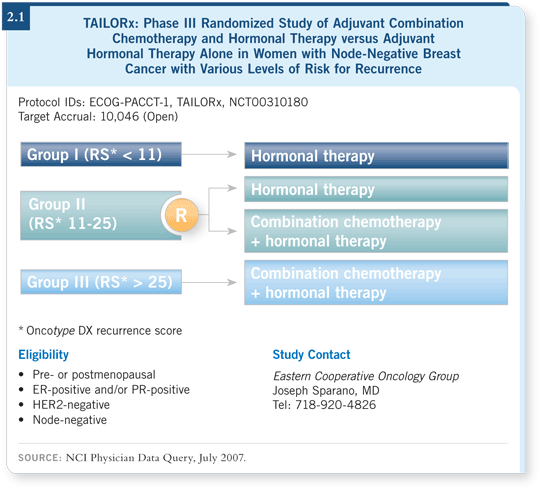
![]() DR HYAMS: The Oncotype DX assay returned a recurrence score of 25, which
means she has approximately a 16 percent risk of developing a distant recurrence
within 10 years. This score placed her in the intermediate-risk category, so she
would be randomly assigned to hormone therapy alone or after chemotherapy,
and she was assigned to chemotherapy followed by hormone therapy (2.1).
DR HYAMS: The Oncotype DX assay returned a recurrence score of 25, which
means she has approximately a 16 percent risk of developing a distant recurrence
within 10 years. This score placed her in the intermediate-risk category, so she
would be randomly assigned to hormone therapy alone or after chemotherapy,
and she was assigned to chemotherapy followed by hormone therapy (2.1).
Her score was at the high end of the intermediate range as defined by the TAILORx criteria, but her score wasn’t quite at the high end of the range specified by the commercially available Oncotype DX assay. The scores for each are assigned a little differently.
Traditional boundaries for the intermediate-risk category, as defined by the commercially available Oncotype DX assay, are 18 and 30 — that is, low risk becomes intermediate risk when the assay score is higher than 18, and a score higher than 30 places a patient in the high-risk category.
For the TAILORx study, the recurrence score range for the intermediate-risk category was lowered to between 11 and 25. The trial designers strongly felt that if randomization methodology were to be employed and therapies prescribed or proscribed, the most conservative approach should be used to help ensure that patients who might benefit from chemotherapy would be eligible to receive it.
Track 10
![]() DR LOVE: Once the chemotherapy has been completed, what about
hormonal therapy?
DR LOVE: Once the chemotherapy has been completed, what about
hormonal therapy?
![]() DR HYAMS: Postmenopausal women have two basic choices: Either tamoxifen
or one of the aromatase inhibitors. The data have increasingly demonstrated
a more favorable toxicity profile for the aromatase inhibitors, with regard to
serious events, and approximately a 20 percent relative risk reduction compared
to tamoxifen.
DR HYAMS: Postmenopausal women have two basic choices: Either tamoxifen
or one of the aromatase inhibitors. The data have increasingly demonstrated
a more favorable toxicity profile for the aromatase inhibitors, with regard to
serious events, and approximately a 20 percent relative risk reduction compared
to tamoxifen.
![]() DR LOVE: What about the issue of tolerability with regard to day-to-day
quality of life with tamoxifen versus the aromatase inhibitors?
DR LOVE: What about the issue of tolerability with regard to day-to-day
quality of life with tamoxifen versus the aromatase inhibitors?
![]() DR HYAMS: That’s a great question because, on paper, the aromatase inhibitors
appear to have the much better toxicity profile, but in reality, that isn’t
true for all patients. In clinical practice, the arthralgias are bothersome enough
to take a number of women off aromatase inhibitors. In my own practice, in a
community of active women who play tennis and golf, the arthralgias can be
extremely bothersome.
DR HYAMS: That’s a great question because, on paper, the aromatase inhibitors
appear to have the much better toxicity profile, but in reality, that isn’t
true for all patients. In clinical practice, the arthralgias are bothersome enough
to take a number of women off aromatase inhibitors. In my own practice, in a
community of active women who play tennis and golf, the arthralgias can be
extremely bothersome.
If we start them on one aromatase inhibitor and they develop arthralgias, our first move is to determine the severity of the problem. If the patient is particularly concerned, we’ll try another one of the aromatase inhibitors. Often they do much better after switching, for reasons that are not clear. Some women can’t tolerate any of the aromatase inhibitors. In such a circumstance we can go back to tamoxifen.
![]() DR LOVE: Which hormonal therapy do you believe she will receive once she’s
finished with chemotherapy?
DR LOVE: Which hormonal therapy do you believe she will receive once she’s
finished with chemotherapy?
![]() DR HYAMS: I believe this woman will end up on an aromatase inhibitor.
DR HYAMS: I believe this woman will end up on an aromatase inhibitor.
Tracks 11-12
![]() DR LOVE: Since the ATAC data demonstrating an advantage to anastrozole
over tamoxifen came out at the end of 2001, many women are about
to complete five years of an aromatase inhibitor. What are your thoughts
on the treatment approach for these patients?
DR LOVE: Since the ATAC data demonstrating an advantage to anastrozole
over tamoxifen came out at the end of 2001, many women are about
to complete five years of an aromatase inhibitor. What are your thoughts
on the treatment approach for these patients?
![]() DR HYAMS: I believe this has to be tested. It’s important to remember that
after five years, a woman has a constant risk of recurrence that runs cumulatively
somewhere between two and four percent per year. Taken in aggregate
over 10 years, that risk is actually significantly higher than the risk for women
who entered any of the prevention trials, P-1 or P-2 (Vogel 2006; Fisher
2005).
DR HYAMS: I believe this has to be tested. It’s important to remember that
after five years, a woman has a constant risk of recurrence that runs cumulatively
somewhere between two and four percent per year. Taken in aggregate
over 10 years, that risk is actually significantly higher than the risk for women
who entered any of the prevention trials, P-1 or P-2 (Vogel 2006; Fisher
2005).
The question is being evaluated in the NSABP-B-42 trial, in which patients who have been treated for five years with an aromatase inhibitor or tamoxifen followed by an aromatase inhibitor will be randomly assigned to either continuation with an aromatase inhibitor or placebo. That trial will provide us with valuable information.
An aromatase inhibitor is not an agent that interacts directly with the cancer cell; it blocks the availability of the agonist. Therefore, it is unlikely that continuing treatment beyond five years is going to be worse, unless there is a long-term toxicity unrelated to its effect on the cancer, of which we are unaware. If a protective effect occurs, we would expect it to continue, as would any mechanism to eliminate or absorb estrogen production.
| Table of Contents | Top of Page |
Editor:
Neil Love, MD
Interviews
Melvin J Silverstein, MD
- Select publications
David M Hyams, MD
- Select publications
Charles E Geyer Jr, MD
- Select publications
Hope S Rugo, MD
- Select publications
Highlights of a CME Symposium Held in Conjunction
with The American Society of Breast Surgeons Eighth
Annual Meeting
- Select publications
A CME Audio Series and Activity