
 |
||||||||||||||

| Tracks 1-18 | ||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 5
![]() DR LOVE: Where are we right now in terms of adjuvant endocrine
therapy for postmenopausal women?
DR LOVE: Where are we right now in terms of adjuvant endocrine
therapy for postmenopausal women?
![]() DR DICKLER: I tend to use an aromatase inhibitor up front with my
postmenopausal patients. I believe the aromatase inhibitors are a little better than tamoxifen. You can prevent some of the earlier events with the aromatase
inhibitors, and I like to use my best drug going forward.
DR DICKLER: I tend to use an aromatase inhibitor up front with my
postmenopausal patients. I believe the aromatase inhibitors are a little better than tamoxifen. You can prevent some of the earlier events with the aromatase
inhibitors, and I like to use my best drug going forward.
I am still interested in defining a sequencing strategy and anxiously await the results of BIG 1-98 (BIG 1-98 2005; Coates 2007) to see if the sequence of an AI to tamoxifen may be better than an AI alone for five years. But, until I know otherwise, I tend to start with an aromatase inhibitor up front.
Track 7
![]() DR LOVE: Another question is the duration of aromatase inhibitors in the
adjuvant setting. Past trials have gone up to five years, and now we have
trials that go beyond five years. Right now, in a clinical setting, how do
you approach the issue of when to stop an aromatase inhibitor?
DR LOVE: Another question is the duration of aromatase inhibitors in the
adjuvant setting. Past trials have gone up to five years, and now we have
trials that go beyond five years. Right now, in a clinical setting, how do
you approach the issue of when to stop an aromatase inhibitor?
![]() DR DICKLER: It is easiest to make these decisions for patients with node-positive
disease, for whom we’ve seen a survival benefit with extended
adjuvant therapy with letrozole (Goss 2005). They are the patients who are
most likely to benefit. With close monitoring of their bone density, I don’t see
much of a downside to continuing, in that I’m not concerned that an aromatase
inhibitor may be detrimental the way we think tamoxifen might be detrimental
after more than five years. Ultimately, talking with the patient and
weighing the risks and benefits is important.
DR DICKLER: It is easiest to make these decisions for patients with node-positive
disease, for whom we’ve seen a survival benefit with extended
adjuvant therapy with letrozole (Goss 2005). They are the patients who are
most likely to benefit. With close monitoring of their bone density, I don’t see
much of a downside to continuing, in that I’m not concerned that an aromatase
inhibitor may be detrimental the way we think tamoxifen might be detrimental
after more than five years. Ultimately, talking with the patient and
weighing the risks and benefits is important.
Where we struggle, however, is with a patient who has node-negative disease, whose risk of a recurrence is lower and who may have comorbid problems. I find that a much more difficult decision. I talk with each patient, but I would, for select patients, offer more than five years of an aromatase inhibitor if I thought they might benefit.
It was fascinating to see from the Overview analysis how many recurrences happen after year five. I don’t believe we fully understood that before. We all have patients who have relapsed 10 and even 20 years out, but seeing that just as many patients relapse after five years as they do in the first five years makes us realize it truly is a chronic disease.
The MA17 trial not only showed us that patients experience recurrence during that period but that we can prevent those recurrences. I believe that was a practice-changing study because now we can affect the natural history of this disease five to 10 years out and maybe more (Goss 2003, 2005).
Patients are being randomly reassigned for 10 versus 15 years. We could possibly be considering 15 years of endocrine therapy, and if we could improve survival, that’s important.
![]() DR LOVE: Most recently the NSABP reported on exemestane after five years
of tamoxifen (Mamounas 2006), which demonstrated a benefit also.
DR LOVE: Most recently the NSABP reported on exemestane after five years
of tamoxifen (Mamounas 2006), which demonstrated a benefit also.
![]() DR DICKLER: It was interesting that 45 percent of the patients crossed over
from placebo to exemestane, and yet there was still a reduction in the hazard ratio. I believe it shows the potency of the aromatase inhibitors and that
estrogen suppression is a useful therapy.
DR DICKLER: It was interesting that 45 percent of the patients crossed over
from placebo to exemestane, and yet there was still a reduction in the hazard ratio. I believe it shows the potency of the aromatase inhibitors and that
estrogen suppression is a useful therapy.
Tracks 13-14
![]() DR LOVE: Can you provide an overview of the available clinical trial data
on the use of trastuzumab in the adjuvant setting?
DR LOVE: Can you provide an overview of the available clinical trial data
on the use of trastuzumab in the adjuvant setting?
![]() DR DICKLER: Trastuzumab in the adjuvant setting has been the greatest
advance we’ve had in the treatment of breast cancer (2.1). The combined
NSABP trial B-31 and Intergroup N9831 study evaluated an anthracycline and
taxane-based regimen (Romond 2005). The Intergroup study evaluated
AC followed by weekly paclitaxel.
DR DICKLER: Trastuzumab in the adjuvant setting has been the greatest
advance we’ve had in the treatment of breast cancer (2.1). The combined
NSABP trial B-31 and Intergroup N9831 study evaluated an anthracycline and
taxane-based regimen (Romond 2005). The Intergroup study evaluated
AC followed by weekly paclitaxel.
The NSABP study was conducted a bit differently. They administered every three-week paclitaxel. They merged these studies and combined the results. The arm that contained trastuzumab clearly showed a tremendous reduction in the risk of recurrence and an improvement in overall survival.
The Europeans designed a different type of study that is a great addition to our treatment regimen (Piccart-Gebhart 2005; Smith 2007). Patients were randomly assigned to trastuzumab versus no trastuzumab after completion of their surgery, radiation therapy and chemotherapy. So it was a sequencing of the targeted agent after chemotherapy. They also showed a powerful reduction in the risk of recurrence and an improvement in survival.
![]() DR LOVE: One regimen in particular in the BCIRG 006 trial — TCH
(docetaxel, carboplatin and trastuzumab) — seems to have a lot less cardiac toxicity and may be as effective as the anthracycline-containing regimens. What are your thoughts on that?
DR LOVE: One regimen in particular in the BCIRG 006 trial — TCH
(docetaxel, carboplatin and trastuzumab) — seems to have a lot less cardiac toxicity and may be as effective as the anthracycline-containing regimens. What are your thoughts on that?
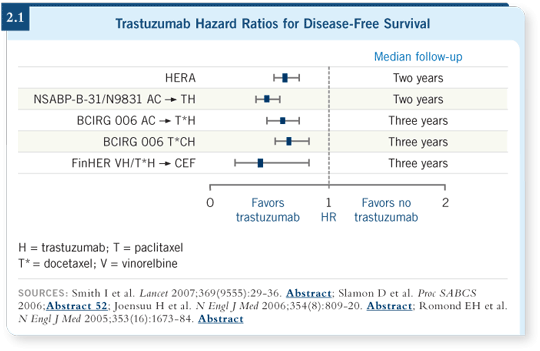
![]() DR DICKLER: Although no statistically significant difference appeared between
the two trastuzumab-containing arms, it was suggested that the AC
DR DICKLER: Although no statistically significant difference appeared between
the two trastuzumab-containing arms, it was suggested that the AC ![]() TH
arm may have performed a little better than the TCH arm in the first interim
analysis (Slamon 2005). The TCH arm now seems to have caught up and seems
to be as good as the AC
TH
arm may have performed a little better than the TCH arm in the first interim
analysis (Slamon 2005). The TCH arm now seems to have caught up and seems
to be as good as the AC![]() TH arm on the second analysis (Slamon 2006).
TH arm on the second analysis (Slamon 2006).
| Table of Contents | Top of Page |
Editor:
Neil Love, MD
Interviews
J Michael Dixon, MD
- Select publications
Maura N Dickler, MD
- Select publications
William C Wood, MD
- Select publications
John Mackey, MD
- Select publications