|
| Genetic Analysis of Tumor Cells and Their
Protein Expression Emmanuel Petricoin, M.D., Co-Director, FDA-NCI
Clinical Proteomics Program, Bethesda, MD |
 |
The field of molecular medicine is moving beyond genomics to proteomics.
While DNA is the information archive, proteins do all the work of
the cell - and ultimately dictate all biological processes and the
cellular fate. The challenge and opportunity within proteomics is
much more than just developing a list of all the proteins. The true
scientific goal of proteomics is to characterize the information
flow within the cell and the organism. This information flow is
mediated through and by, protein pathways and networks.
The cause of most human disease lies in the functional disregulation
of protein-protein interactions. Understanding the role that protein
networks play in disease will create enormous clinical opportunities,
since these pathways represent the drug targets of the next decade.
In the future, entire cellular networks, not just one disregulated
protein, will be the targets of therapeutics. The next technologic
leap will be the application of proteomic technologies to the bedside.
It will soon be possible to analyze the state of protein signal
pathways in the disease-altered cells, before, during, and after
therapy. This can herald the advent of true patient-tailored therapy.
Our program is focused on the understanding of mechanisms of carcinogenesis,
identification of new drug targets, and discovery of new biomarkers
for early detection in actual human tissue tumor specimens. Tissue-based
proteomics requires technology that can overcome the complex cellular
heterogeneity one encounters when studying disease in tissue specimens.
To that end, we employ the use of Laser Capture Micro-dissection
(LCM) for the proteomic analysis of microdissected subpopulations
of human solid tumors (prostate, breast, ovary, and esophageal)
as a model for the study of disease progression. These studies encompass
and employ:
a. Differential protein profiling and discovery technologies, such
as two-dimensional polyacrylamide gel electrophoresis (2D-PAGE)
coupled with mass spectrometry for new target and biomarker discovery-
over 140 protein have been identified to date.
b. High-throughput proteomic pattern profiling using surface-enhanced
laser desorption and ionization (SELDI) to identify disease-related
proteins and protein patterns and
c. Focused proteomic approaches through the use of multiplexed phospho-specific
antibody arrays, and general antibody and lysate arrays for signal
transduction pathway profiling.
| Signal Transduction Inhibitors as Anti-Neoplastic
Agents Clifford Hudis, M.D., Chief, Medical Oncology of Breast
Center, Memorial Sloan-Kettering Cancer Center, New York City,
NY |
 |
The growth in understanding of the underlying mechanisms and pathways
contributing to malignant transformation and tumor progression is
fueled in large part by the expectation that this knowledge will
lead to improved therapeutics. This hope is based on successes in
the past beginning most notably with hormone therapy which was the
first targeted therapy in oncology. A variety of chemotherapy agents
have been developed with some degree of target specificity but increasingly
the exact mechanisms of cytotoxicity are questioned. Very recently
targeted therapy in general has been energized by the availability
of several active monoclonal antibodies including trastuzumab (Herceptin).
The exact mechanism underlying the activity of trastuzumab is still
debated and may include cell mediated immunity. However, inhibition
of signal transduction through its prevention of HER family heterodimerization
appears to be a key activity of this agent. When this signal is
prevented a variety of downstream events are seen, cell growth is
inhibited, and apoptosis ensues. Because this targeted therapy offers
the potential for improved efficacy with minimized toxicity there
is now a growing list of drugs under development which may be even
more effective. Examples include drugs targeting the tyrosine kinase
activity associated with HER2 or other members of the HER family
(ie, HER1 or EGF-R), as well as drugs aimed at other non-HER kinases.
Ongoing clinical trials are exploring the role of relatively specific
EGF-R inhibitors, such as OSI-74 and ZD 1839 (Iressa) as well as
other non-HER targeting agents, such as imatinib mesylate (Gleevec).
From these trials we should learn not only about the potential efficacy
of these agents but also more about the underlying molecular biology.
| Targeting the epidermal and HER2/neu growth
factor receptor Debu Tripathy, M.D., UCSF Carol Franc Buck Breast
Care Center, University of California, San Francisco, CA |
 |
Specific genetic, biochemical and physiological events predispostion
and lead to the development of cancer. As these biological pathways
are further understood, targeted therapies that modulate these pathways
are being developed. Several aspects of tumor cell behavior are
amenable to different types of pharmacological manipulation that
could produce a significant clinical advantage
Biological Targets
Types Targeting Agent |
|
- Growth factor receptor signaling system
- Antibodies
- Nuclear receptor systems/transcriptional factors
- Gene therapy
- Cell cycle control proteins (cyclins, cyclin-dependent
kinases (CDKs), CDK inhibitors
- Gene disruption (antisense, ribozymes)
- Programmed cell death (apoptosis) proteins
- Immune cells (dendritic cell vaccines)
|
- Proteases/protease inhibitors
- Small molecule kinase inhibitors
- Angiogenesis (receptors and factors)
- Peptides/cytokines
- DNA/RNA metabolism
- Natural compounds
- Tumor-specific antigens
|
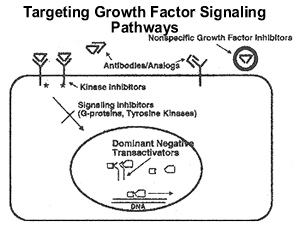 Growth
factor receptors are one of the most relevant and studied targets
in breast and certain other cancers. The epidermal growth factor
receptor family consists of the epidermal growth factor receptor
(HER1/erbB-1), HER2/neu (HER2, erbB-2), HER3 and HER 4. HER2/neu
has been extensively characterized and targeted, leading to the
approval of trastuzumab (Herceptin) as the first biological therapy
for breast cancer. See the syllabus entitled “Herceptin as
single agent and/or combination with cytotoxic or hormonal agents”
for details. Growth
factor receptors are one of the most relevant and studied targets
in breast and certain other cancers. The epidermal growth factor
receptor family consists of the epidermal growth factor receptor
(HER1/erbB-1), HER2/neu (HER2, erbB-2), HER3 and HER 4. HER2/neu
has been extensively characterized and targeted, leading to the
approval of trastuzumab (Herceptin) as the first biological therapy
for breast cancer. See the syllabus entitled “Herceptin as
single agent and/or combination with cytotoxic or hormonal agents”
for details.
This diagram illustrates the signal generated by the interaction
of ligand and growth factor receptor and the potential elements
that can be modulated therapeutically. Numerous downstream effects
that can be seen with receptor signaling, primarily mitogenesis,
but also changes in motility, metabolism, and even paradoxically,
enhancement of programmed cell death can all be seen.
Along with the HER2/neu receptor, the epidermal growth factor
receptor (EGFR) has also been a target of interest. EGFR is overexpressed
in lung and head/neck cancers as well as a proportion of colon and
breast cancer. A monoclonal antibody to EGFR, C225, has activity
against EGFR expressing cancer cells in vitro. Human trials that
have shown promise include C225 plus radiation for head and neck
cancer and C225 plus irinotecan in refractory colon cancer. Small
molecule kinase inhibitors that act in a receptor-specific fashion
on the cytoplasmic kinase domain can effectively squelch the downstream
signaling mediated by the receptor. Two such agents, ZD 1689 (Iressa),
and OSI-774 (Tarceva) have shown some activity in lung cancer or
head and neck cancer. Since breast cancer cells also express EGFR
and since EGFR may heterodimerize with HER2/neu, there are ongoing
trials testing these agents in breast cancer. Combinations of these
small molecule inhibitors with hormonal therapy are also being tested
in breast cancer. Given the potential heterodimerization of HER2/neu
and EGFR, a trial of Iressa plus Herceptin is also planned.
The vascular endothelial growth factor receptors (VEGFR-1 and
VEGFR-2) mediate angiogenesis and vascular permeability and may
have an important role in tumor growth and metastasis. Targeting
these growth factor receptors have an anti-angiogenic effect in
animal models and are also being tested in the clinic. In a small
phase II study of anti-VEGF antibody for advanced breast cancer,
a small number of responses were seen with side effects including
hypertension and proteinuria. Currently, this agent is being tested
in combination with capecitabine or in combination with paclitaxel
in two separate randomized trials comparing the combination to chemotherapy
alone. Small molecule inhibitors to VEGFR are also being tested
in breast and other cancers.
Components of the receptor signaling pathways that may predict
responsiveness to such therapies are of great interest since these
may optimally choose patients who have a good chance of response.
It is unlikely that any single agent will have a dramatic effect
on breast cancers universally since a given pathway may not be operative
in all cases of cancer. Hence, predictive markers may not only help
select the type of drug to be employed in a given patient, but may
also identify novel therapeutic targets.
| Evolution of Premalignant Lesions from Normal
Epithelium to DCIS D. Craig Allred, M.D., Professor of Pathology,
Breast Center, Baylor College of Medicine, Houston, TX |
 |
Most (probably all) invasive breast cancers (IBCs) arise from certain
pre-existing benign lesions over long periods of time. There are
many types of benign lesions in the breast and only a handful appears
to have significant premalignant potential. The most important premalignant
lesions include hyperplastic unfolded lobules (HULs), atypical ductal
hyperplasia (ADH), atypical lobular hyperplasia (ALH), ductal carcinoma
in situ (DCIS), and lobular carcinoma in situ (LCIS). Several converging
lines of pathological, epidemiological, and biological evidence
support the premalignant nature of these lesions including: (1)
they are on histological continuum, (2) they are much more common
in cancerous than non-cancerous breasts, (3) they are risk factors
for developing IBC, and (4) they share identical genetic and epigenetic
defects with synchronous IBC in the same breast. Currently, premalignant
lesions are defined by their histological features. By definition,
lesions within specific categories look alike under the microscope
but only a relatively small proportion appear to progress to IBC,
emphasizing that there must be underlying biological differences
causing some to remain stable and others to progress. Understanding
this biology has become an important topic in cancer research and
there have been a large number of publications in the literature
over the past decade. Nearly all have been descriptive or correlative
studies of archival (i.e. formalin-fixed and paraffin-embedded)
clinical tissue samples because few if any cell lines or animal
models exist to support mechanistic studies. Despite these limitations,
a great deal has been learned about biological phenomena that appear
to be important in the evolution of premalignant disease including
growth kinetics (proliferation and apoptosis), the estrogen receptor
(ER), oncogenes (e.g. erbB2), tumor suppressor genes (e.g. p53),
allelic imbalances, and so on. Alterations in ER expression, structure,
and function appear to be particularly important in the early development
of premalignant lesions from normal cells and will be mentioned
in more detail during the presentation. However, like fully developed
IBCs, premalignant lesions as a whole are almost certain to contain
a large number of as yet unknown critical biological abnormalities
which, hopefully, some of the new high-throughput technologies will
help unravel. Understanding this biology is an important goal because
it may help identify new prognostic and predictive factors to improve
the treatment of patients with premalignant disease and, more importantly,
it may identify new therapeutic targets for breast cancer prevention.
| Transition from normal-hyperplasia-DCIS-invasion
and metastasis using Laser capture microdissection. Clinical
implications Emmanuel Petricoin, M.D., Co-Director, FDA-NCI
Clinical Proteomics Program, Bethesda, MD |
 |
The study of the biological changes that underpin the transition
from normal through frank breast cancer has been the focus of intensive
genetic studies for the past decade or more. However, since the
mechanisms by which the cancer cell arises and survives are ultimately
dictated by protein function, proteomics offers a new opportunity
to study carcinogenesis. However, because the proteome is in a constant
state of flux, and is cell-type and context dependant, simply grinding
up a human tumor specimen and analyzing the protein content will
not yield the information sought. Cellular heterogeneity confounds
these types of analysis as the proteome of stromal cells, infiltrating
lymphocytes, normal, DCIS, and cancer epithelial cells cross-contaminate
each other. Consequently, we have developed and utilized Laser Capture
Microdissection (LCM) to procure pure homogeneous populations of
cells directly from breast cancer human tissue specimens to study
the protein changes that occur as the transition from normal to
frank cancer occurs. Proteomic technologies such as 2D-PAGE separation
followed by mass spectroscopy, and implementation of new types of
protein arrays are coupled to LCM for discovery of new proteins
that may be therapeutic targets or biomarkers for early detection
and staging.
| Angiogenesis Inhibition; Conceptual Framework
and Challenges Kathy D. Miller, M.D., Associate Professor of
Medicine, Indiana University, Indianapolis, IN |
 |
Angiogenesis, the process of new blood vessel formation, plays
a central role in both local tumor growth and distant metastasis.
Angiogenesis is a tightly regulated, multiply redundant process
required only for wound healing, endometrial proliferation, and
pregnancy in healthy adults. Thus the inhibition of angiogenesis
offers an attractive therapeutic target with little expected (at
least theoretically) toxicity. Rather than a comprehensive review
of all agents currently in development, we can conceptually group
agents into several categories based on the mechanism of action:
protease inhibitors which either directly inhibit or otherwise interfere
with the action of proteases critical for invasion, growth factor/receptor
antagonists which thwart signaling of pro-angiogenic growth factors,
endothelial toxins which specifically target endothelial antigens,
and natural inhibitors which stimulate or mimic substances known
to naturally inhibit angiogenesis. The clinical experience with
each category and potential barriers will be reviewed.
| NSABP Chemotherapy Prevention Trials. Richard
Margolese, M.D., Herbert Black Professor of Surgical Oncology,
McGill University, Montreal, Canada |
 |
The traditional model for attacking breast cancer has been to detect
the cancer at an earlier stage when it is more curable and to add
adjuvant therapies to improve cure rates after surgery. A useful
model of breast cancer suggests that DCIS is a pre cursor of invasive
cancer and other changes such as atypical hyperplasia are even earlier
changes that frequently lead to cancer. Intervening to interrupt
the evolution of these changes to invasive cancer with the potential
for metastasis is a logical and useful therapy.
In two randomized clinical trials of DCIS treatment, NSABP protocols
have shown that lumpectomy with post operative radiation and tamoxifen
therapy reduced the rate of invasive breast cancer to approximately
2%
In other reports survival after mastectomy for DCIS varies from
98-99%.
In addition, tamoxifen has shown in the adjuvant therapy setting
that it can inhibit the appearance of new cancers in the contralateral
breast. This led to the design and completion of a prevention trial
involving 13,600 high risk participants. Therapy with tamoxifen
reduced breast cancer risks by 50% with acceptable levels of side
effects. These two trials illustrate the possibility of controlling
the evolution of breast cancer with SERMs which can be considered
as growth factor inhibitors. Future research should expand and extend
these findings for better control of breast cancer incidence.
hmemon/haldoc/abstract\osman02.abs
| Results of the ATAC trial (Arimidex, Tamoxifen
and Combination). Implications for chemoprevention. Professor
Anthony Howell, CRC Department of Medical Oncology, University
of Manchester, UK |
 |
The new non-steroidal and steroidal aromatase inhibitors have been
shown to be superior to megestrol acetate as second line endocrine
therapy and to atamoxifen for first line endocrine therapy for advanced
breast cancer. The first adjuvant trial to be reported using an
aromatase inhibitor (anastrozole) now indicates that aromatase inhibitors
may become the treatment of choice in the adjuvant situation and
thus this leads to the suggestion that they may be of value in breast
cancer prevention. Anastrozole is a highly selective potent aromatase
inhibitor which is non-steroidal and orally active. It is given
as a once daily dose (1mg) and there are now over 460,000 patients
years experience with this drug. The ATAC trial was initiated approximately
5.5 year’s ago and compares anastrozole 1mg plus tamoxifen
placebo versus anastrozole placebo plus tamoxifen 20mg versus a
combination of the two drugs. The primary endpoints of the study
were disease free survival, safety and tolerability. The secondary
endpoints included incidence of new breast (contralateral) primary
cancers. The first results of the trial were given at the San Antonio
Breast Symposium on 10.12.01 (M Baum). Patients were recruited from
21 countries into the trial between July 1996 and March 2000. In
all, 9,366 patients were randomised. Analysis was pre-defined when
1,056 events had occurred. The mean age of this postmenopausal population
was 64 years, 64% of patients had T1 tumours and 34% were node positive.
Eighty four percent of tumours were oestrogen receptor positive
and/or progesterone receptor positive and 9% were receptor unknown.
The remainder were receptor negative. The total number of first
events was 1,079, 766 of which were in the receptor positive population.
The median duration of therapy was 30.7 months and the median follow
up 34.3 months. The Kaplan-Meier curves of disease free survival
in the intention to treat the population showed that anastrozole
was superior to tamoxifen (HR 0.83, 95.2% CI, 0.71-0.96, p0.0129).
The combination was equivalent to tamoxifen (HR 1.02, 95.2% CI,
0.88-1.18, p0.7718). There was a diminution in both locoregional
and distant relapses. Analysis of the incidence of new (contralateral)
breast primaries showed that anastrozole was markedly superior to
tamoxifen (OR 0.52, 95% CI, 0.22-0.79, p0.0068). There was no significant
difference between the combination and tamoxifen. There were significantly
fewer hot flushes, weight gain, vaginal problems and thromboembolic
phenomena in the arimidex arm. However, there were more musculoskeletal
disorders and more fractures in the anastrozole alone arm. Thus,
the early analysis of the ATAC trial shows a disease free advantage
for anastrozole and a marked reduction in the numbers of contralateral
tumours compared with tamoxifen or the combination with an improved
safety profile. These features, particularly the reduction in contralateral
breast cancers, make anastrozole a potentially useful agent for
breast cancer chemoprevention. A trial comparing anastrozole with
tamoxifen and placebo in women at risk of breast cancer is due to
start later this year (IBIS II).
| The Intraductal Approach to the Breast Susan
M. Love, M.D. MBA, Adjunct Professor of Surgery UCLA, Pacific
Palisades, CA |
 |
The idea of studying breast cancer by accessing the milk ducts
is not a new one. Interest in nipple fluid dates back to the early
1900’s. As early as 1914 Nathan reported a case in which breast
cancer was diagnosed on the basis of malignant cells found in nipple
discharge. In 1950 George Papanicolaou started a research project
with an aim “to ascertain the proportion of asymptomatic women
from whom breast secretion could be obtained with a consideration
of the possibility of utilizing breast secretion smears in screening
for mammary carcinoma.” He applied a suction to the nipple
and but was able to obtain fluid from only 18.6% of women. This
approach languished until the 1970’s when several groups studied
this “breast pap smear” . Sartorius reported on a series
of 1503 women from his breast surgical practice and was able to
obtain fluid from 55% of subjects. Using his device Buerhing and
Petrakis reported similar yields in volunteers. Petrakis and Wrensch
contributed greatly to the field over the years by analyzing the
fluid for cells as well as biochemical, hormonal and exogenous substances.
Their recent twenty year follow up confirmed that women who have
atypical cells in their nipple aspirate fluid have a doubling of
their risk of subsequent breast cancer. If they have a family history
of breast cancer and aypia the relative risk is 4.5 . Most recently
Sauter has championed this approach and reported the ability to
obtain fluid from 100% of women with multiple attempts.
The first description of ductal lavage and indeed intraductal
biopsy was by Raul LeBorgne of Uruguay in the early 1940’s.
He describes cannulating the milk ducts and doing ductograms and
then collecting the contrast material for analysis. This he called
ductal rinse. Sartorius in the 1970’s continued this practice
and in 1974 suggested that “ perfusion of the non-productive
ducts with isosmotic, sterile saline solution via nylon catheters
followed by nipple aspiration, is a ‘cell washing” technique
found to be extremely effective in procuring cells from the terminal
ramifications of the duct structure.” Of 27 women suspected
of having carcinomas on cytology, eighteen were found to have cancer.
Seven of these were identified on the basis of cytology alone. All
of the lesions were smaller than 8mm.In 49 cases of carcinoma diagnosed
by another modality no fluid could be aspirated from the breast.
Sartorius postulated that larger tumors had disrupted the ducts
and that the fluid was no longer contained.
It was this work of Sartorius which led me to explore the concept
of ductal lavage. Both Sartorius and LeBorgne had instilled fluid
into the ducts and then removed the catheter and aspirated the fluid
they squeezed out of the nipple from its surface. In order to be
sure which duct was abnormal, I felt we needed a technique to cannulate
a single duct and aspirate the fluid from that duct. The ProDuct
catheter was developed on that premise. The current application
of ductal lavage has been presented already by Darius Francescatti
and will be reviewed in the satellite session this evening.
Parallel to the ductal lavage development has been the exploration
of ductoscopy. First described by Teboul in association with ultrasound
of the ductal lobular units, this ability to directly visualize
the lining of the ducts takes the intraductal approach to another
level. Several researchers in Japan, the US, Spain, Germany and
Argentina have employed ductoscopy in women with spontaneous nipple
discharge. Our early limited work with this technique in non discharging
ducts has been followed by a report from Dooley in Oklahoma, who
has used ductoscopy to investigate abnormal findings on lavage.
The ability to access the ductal systems has great potential to
help in our understanding of the physiology of the non lactating
breast. The non lactating breast appears to be metabolically active.
It has immunoglobulins, proteins, carbohydrates and significant
levels of cholesterol and its metabolites at different levels than
those found in the blood. Breast duct fluid has been shown to contain
nicotine within a half an hour of a woman smoking a cigarette. Small
studies have demonstrated PSA, CEA, LDH, P 53, and Her 2 neu in
the fluid. Sukumar’s group has demonstrated the ability to
detect methylation of genetic markers in breast duct fluid and the
NCI has demonstrated the ability to perform mutagenesis assays,
amplify DNA and perform protein electrophoresis on nipple aspirate
fluid.
The microenvironment of the ductal lobular system is undoubtedly
an important factor in the development of breast cancer. Estrogen,
testosterone, prolactin, progesterone, and dehydroepiandrosterone
sulfate have been found in the breast fluid. Interestingly hormone
levels in the fluid are independent of the blood. In premenopausal
women the levels of estrogen do not reflect the menstrual period
but are much higher. Petrakis found that they go down with pregnancy
and breast feeding and then gradually climb over the next four years.
They remain high in postmenopausal women even after serum levels
have dropped and can be as much as forty times higher. This may
reflect the presence of aromatase in the breast ducts. A report
from Harding et al demonstrated that proteins normally stimulated
or inhibited by estrogen could be measured in the breast duct fluid
and be used to demonstrate the effects of Tamoxifen, HRT and goserelin.
We have made significant progress in understanding and treating
cancers of the cervix and colon, in part because we have had access
to where they start. It is my hope that the intraductal approach
to the breast will lead to equivalent advances.
Selected references:
- Love SM, Barksy Sh Breast-duct Endoscopy to Study Stages of
Cancerous Breast Disease Lancet (1996) 348:997-9.
- Petrakis NL Physiologic, Biochemical, and Cytological Aspects
of Nipple Aspirate Fluid. Breast Cancer Res Treat (1986) 8:7-19.
- Phillips HA, Howard GCW, Miller WR Nipple aspirate fluid in
relation to breast cancer: A Review. The Breast (1999) 8, 169-174.
- Sartorius OW, Smith HS, Morris P, Benedict D, Friesen L Cytologic
Evaluation of Breast Fluid in the Detection of Breast Disease
J Natl Cancer Inst (1977) 59:1073-1080
- Wrensch MR, Petrakis NL, Miike R, King EB, Chew K, Neuhaus
J, Lee MM, Rhys M Breast Cancer Risk in Women With Abnormal Cytology
in Nipple Aspirates of Breast Fluid J Natl Cancer Inst (2001);93:1791-8.
| Ultrasound-guided needle biopsy of non-palpable
breast masses Bruno D. Fornage, M.D., Professor of Radiology
& Surgical Oncolory, M.D. Anderson Cancer Center, University
of Texas, Houston, TX |
 |
Because of its unique real-time capability, sonography (US) has
become the preferred modality for guiding needle biopsy of nonpalpable
breast masses. Virtually any nonpalpable breast lesion that is clearly
seen on sonograms can be sampled with a needle under US guidance.
Both fine-needle aspiration biopsy (FNA) and core-needle biopsy
(CNB) are effectively guided by real-time US.
Fine-Needle Aspiration
Standard 20-gauge, 1.5-inch (3.8-cm) hypodermic needles are used
for FNA. Rarely, a 2-inch (5-cm) needle is needed because the lesion
is deep and/or the breast is large. The standard technique of needle
insertion for FNA is oblique insertion, in which the needle is inserted
from the end of the transducer along the scan plane with an obliquity
that depends on the depth of the target. In experienced hands, the
oblique insertion technique is 100% accurate and safe.
Cysts and other fluid collections can be readily aspirated with
a fine needle. On occasion, an 18-gauge needle may be needed to
drain an inspissated cyst, which typically contains toothpaste-like
material. In such a case, it may not be possible to drain the cyst
completely. Other collections that can be subjected to diagnostic
FNA or percutaneous drainage in the proper clinical setting include
postoperative hematomas, lymphoceles, and abscesses.
FNA of infiltrating ductal carcinomas usually yields highly cellular
cytologic specimens; with US guidance, the diagnosis is established
with a single pass in the majority of cases. Other forms of breast
malignancy, such as medullary or mucinous carcinomas, lymphomas,
or metas-tases to the breast from extramammary primary cancers,
can also be correctly diagnosed cytologically. Hormonal receptors
and proliferation markers including Her-2/Neu can be assessed in
fine-needle aspirates from breast cancer, although this is usually
done on CNB specimens. With strict criteria, the diagnosis of fibroadenoma
can be reliably established cytologically. FNA can also readily
establish the diagnosis of fat necrosis, acute inflammation, or
intramammary lymph nodes.
The major limitation of FNA is its operator dependence. When FNA
specimens are insufficient, the operator should switch to CNB.
Core-Needle Biopsy
Numerous commercially available devices provide automatic propulsion
of a cutting needle with a throw of about 2 cm. Because of the throw
of the needle, the cutting needle must be inserted as horizontally
as possible to avoid any injury to the chest wall. When the transfixion
of the target has been clearly documented with US and cores appear
to be of satisfactory size, no more than three or four cores are
needed for diagnosis.
In rare cases, US-guided CNB is done to sample an area of microcalcifications
without a discrete mass. In this case, it is imperative to process
the cores the same way they would be if the biopsy were done under
stereotactic guidance, i.e., the cores obtained must be radiographed
using a mammographic unit and magnification technique (or dedicated
equipment for specimen radiography) to document the presence of
the calcifications in the cores.
Other Biopsy Devices That Can Be Used under US Guidance
A handheld version of the 11-gauge Mammotome device (Ethicon Endosurgery)
has been designed for use with US guidance. Advantages of the device
include a single insertion of the device, convenient automatic core
retrieval, and multiple large contiguous samples with the potential
to remove small masses entirely. In the United States, the device
has been approved for biopsy, but not for therapeutic purposes.
Disadvantages of the Mammotome include the large diameter of the
cutting device, the greater associated trauma, and the relatively
high cost.
A new breast biopsy device, en-bloc(tm) (Neothermia), has been
designed to cut with radiofrequency and retrieve a large (1-cm diameter)
intact specimen with a capture snare. While such large-bore biopsy
devices and the large volume of the cores they yield are well adapted
to stereotactically guided biopsy of microcalcifications, their
cost-effectiveness compared with standard Tru-cut needles for the
biopsy of breast masses remains to be demonstrated.
Keys to Success of US-Guided Needle Biopsy
US-guided needle biopsy of nonpalpable breast lesions requires teamwork.
Progress can be made and experience accumulated only through ongoing
communication between the radiologist, the pathologist, and the
surgeon. US-guided biopsy procedures require excellent eye-hand
coordination and a significant amount of practice before the mandatory
100% accuracy level in hitting the target can be reached. Practicing
with easy-to-make phantoms shortens the learning curve of beginners.
The golden rule in breast biopsy is the concordance between the
results of a biopsy and the imaging and clinical findings. Any discrepancy,
e.g., a negative result of the needle biopsy in the face of a single
suspicious finding-physical, mammographic, or US-should not delay
surgical excision.
References
- Fornage BD, Faroux MJ, Simatos A. Breast masses: US-guided fine-needle
aspiration biopsy. Radiology 1987;147:409-414.
- Fornage BD, Sneige N, Faroux MJ, et al. Sonographic appearance
and ultrasound-guided fine-needle aspiration biopsy of breast
carcinomas smaller than 1 cm3. J Ultrasound Med 1990;9:559-568.
- Fornage BD, Coan JD, David CL. Ultrasound-guided needle biopsy
of the breast and other interventional procedures. Radiol Clin
North Am 1992;30:167-185.
- Fornage BD. Ultrasound-guided percutaneous needle biopsy on
nonpalpable breast masses. In: Harris JR, Lippman ME, Morrow M,
Hellman S (eds). Diseases of the breast. Philadelphia, Lippincott-Raven,
1996, pp. 152-158.
- Fornage BD. Sonographically guided needle biopsy of nonpalpable
breast lesions. J Clin Ultrasound 1999;27:385-398.
- Fornage BD, Sneige N, Edeiken BS. Interventional breast sonography.
Eur J Radiol, in press.
- Parker SH, Jobe WE, Dennis MA, et al. US-guided automated large-core
breast biopsy. Radiology 1993;187:507-511.
| MRI in Planning DCIS Management Steven E.
Harms, MD, FACR, Professor of Radiology, University of Arkansas
for Medical Sciences, Little Rock, AR, and Medical Director,
Aurora Imaging Technology, North Andover, MA |
 |
Although high sensitivity is a common attribute in almost all breast
MRI papers, the gold standard for this validation is highly variable.
Most breast cancer studies used biopsy as a gold standard. With
this methodology, only the lesion actually sampled is confirmed.
The ability to detect cancer in the breast that is not sampled with
the biopsy is not possible. Therefore, this method inherently underestimates
false negative breast MRI findings. We know that routine pathology
misses additional cancers in 40% of breasts.(1-3) Therefore, in
order to truly measure for the potential of false negative breast
MRI, mastectomy specimens must be serially sectioned and rigorously
analyzed by pathology. This analysis is limited to only a few MRI
studies.(4) Without this validation, the true negative predictive
value of breast MRI is not known.
Although it has not been proven with serial section mastectomy
correlations, it is likely that dynamic, low-resolution breast MRI
misses cancer. Most of the reported previously reported studies
show an under-reporting of DCIS. Many of these studies cite less
than 10% DCIS in the population studied.(5-7) Recent screening mammography
experiences report up to 40% representation by DCIS. These data
indicate that dynamic, low-resolution breast MRI is missing DCIS.
Many experts now conclude that dynamic, low-resolution breast MRI
cannot reliably detect DCIS and only cite specificity statistics
for infiltrating carcinoma.(8) A major potential limitation of breast
MRI is the inability to detect DCIS. This limitation is solvable
with high contrast, high resolution images.
High resolution, fat-suppressed breast MRI has been validated using
serial section mastectomy analysis for a proven negative predictive
value approaching 100%. We evaluated RODEO MRI for the ability to
detect DCIS and found it to be highly effective.(9) Because RODEO
uses narrow bandwidth RF, microcalcifications can be seen as tiny
foci of magnetic susceptibility effect intermixed with the typical
clumped enhancement of DCIS.
Because of the high quality definition of DCIS by RODEO MRI, we
are now using this information in combination with stereotaxic localization
to mark the margins of DCIS extent prior to surgery. DCIS typically
does not involve a spherical volume. DCIS usually extends within
the ductal system involving a segment or adjacent segments. The
ducts are arranged radially extending from the nipple to the chest
wall. Therefore, a surgical excision that removes a spherical volume
of tissue may likely result in positive margins. RODEO MRI can identify
the margins of the DCIS extent for better resections.
Localization wires are a problem for MRI. Although MRI compatible
wires are abundant, the access to the MRI relative to surgical procedures
is often difficult. We have many patients from other institutions,
even out-of-state who are referred for localization. Leaving a wire
in place for a long enough period to travel to distant hospitals
for surgery is inadvisable. For this reason we developed another
system that is working well..
Instead of wire localizations, we typically perform hematoma localizations.
For this procedure needles are placed around the lesion to mark
the margins. The correct identification of margins is confirmed
on a MR image. Approximately 2 cc of the patients’s own blood
is injected into each site. The hematoa shows up as a bright spot
on the immediate MR image after hematoma localization. The hematoma
is identified at surgery with ultrasound and visually when exposed
in the surgical field. No needles are wires are left in place to
risk infection. The hematoma lasts for days. The patients are free
to travel to other institutions without restriction.
References
- Rosen PP, Fracchia AA, Urban JA, et al. “Residual”
mammary carcinoma following simulated partial mastectomy. Cancer.
1975;35:739-47.
- Holland R, Veling SHJ, Mravunac M, Hendriks JHCL. Histologic
multifocality of Tis, T1-2 breast carcinomas: implication for
clinical trials of breast-conserving surgery. Cancer. 1985;56:979-90.
- Holland R, Connolly JL, Gelman R, et al. The presence of an
extensive intraductal component following a limited excision correlates
with prominent residual disease in the remainder of the breast.
J Clin Oncol. 1990;8(1):113-8.
- Harms SE, Flamig DP, Hesley KL, et al. Breast MRI: rotating
delivery of excitation off-resonance: Clinical experience with
pathologic correlations. Radiology 187:493-501, 1993.
- Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging
sequences with and without Gd-DTPA. Radiology. 1989;170:681-6.
- Heywang SH, Wolf A, Pruss E, Hilbertz T, Eiermann W, Permanetter
W. MR imaging of the breast with Gd-DTPA: use and limitations.
Radiology. 1989;171:95-103.
- Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR
imaging: Are signal intensity time course data useful for differential
diagnosis of enhancing lesions? Radiology 211:101-110, 1999.
- Kuhl CK, Schmutzler RK, Leutner CC, Kempe A, Wardelmann E,
Hocke A, Maringa M, Pfeifer U, Krebs D, Schild HH. Breast MR imaging
screening in 192 women proved or suspected to be carriers of a
breast cancer susceptibility gene: preliminary results. Radiology
2000 215: 267.
- Soderstrom CE, Harms SE, Copit DS, et al. 3D RODEO breast MRI
of lesions containing ductal carcinoma in situ. Radiology 201:427-432,
1996.
| Treatment of Ductal Carcinoma in Situ Summary
of the Consensus Conference, Philadelphia, April, 1999. Gordon
F. Schwartz, MD, MBA, Professor of Surgery, Jefferson Medical
College, Philadelphia, PA |
 |
The goal of treatment for DCIS is breast conservation, with optimal
cosmesis and with a minimum risk of subsequent invasive or in situ
recurrence. There are some women for whom mastectomy remains the
optimal treatment, but most women with DCIS are candidates for breast
conservation. Each patient should be apprised of her own situation
and each option discussed with her in detail, including local excision
and radiation therapy, local excision alone, or mastectomy. Regardless
of the goal of breast conservation, mastectomy is an acceptable
treatment options for patients with DCIS, irrespective of their
eligibility for breast conservation. A minority of patients with
DCIS requires mastectomy, probably less than 25% of patients with
this diagnosis, but mastectomy may be performed if it were the patient’s
preference.
The majority of women with DCIS, therefore, are candidates for
breast conservation. Whether radiation therapy, surveillance alone,
or either of these plus tamoxifen, is optimal treatment when breast
conservation is employed, is a major topic of current discussion.
There are groups of patients with DCIS who fall into each of these
categories, but no one has yet defined the selection criteria for
each of them precisely enough to make dogmatic recommendations.
Clinical trials have shown that local excision and radiation therapy
in patients with negative margins provide excellent rates of local
control. Patients treated by excision alone have a greater chance
of local recurrence. This risk decreases with wider surgical margins
around the area of DCIS. Although adding radiation therapy to wide
local excision benefits all groups of DCIS patients who are candidates
for breast conservation, the magnitude of that benefit may be small
enough in some patient sub-groups that radiation can be omitted.
However, patients who may avoid radiation therapy have not been
reproducibly and reliably identified by any clinical trials, but
there are institutional and individual reports of large series of
such patients treated by wide excision alone who achieve a 1% or
less per year risk of invasive recurrence, which is the reported
occurrence of invasive cancer following local excision and radiation.
Clear surgical margins are a major criterion for treatment of DCIS
by breast conservation (whether or not radiation therapy is employed);
the wider the margin, the lower the rate of local recurrence. Margin
status is crucial importance because it is the one variable that
the physician can control. A 10 mm margin the best compromise between
removal of so much tissue that the cosmetic result would be less
than desirable and the likelihood of local recurrence.
An axillary dissection is not required in patients with DCIS. The
incidence of occult invasive carcinoma that might have spread to
axillary nodes is so infrequent that this possibility should not
provoke a therapeutic recommendation to dissect the axilla. For
patients undergoing mastectomy, some lymph nodes situated in the
axillary tail of the breast may be removed incidentally, but an
intentional dissection of the axilla is not warranted.
The role of sentinel node biopsy in DCIS is also a major contemporary
question. In general, sentinel node biopsy should be considered
only in the context of a peer-reviewed clinical trial. Although
sentinel node biopsy, using either radioactive isotope or blue dye
to locate the node(s), is less invasive and less morbid than the
customary axillary dissection performed for invasive cancer, the
ease of sentinel node biopsy should not be an excuse for its use.
If, by definition, DCIS does not spread to lymph nodes, a procedure
to address lymph node status should not be necessary, however free
from complications it might be.
There is no indication for adjuvant cytotoxic chemotherapy in patients
with DCIS. In patients undergoing mastectomy, in the unlikely event
that a focus of invasive carcinoma is found in the specimen, the
patient should be treated as for any other stage I carcinoma of
the breast of the same size.
A randomized clinical trial has demonstrated that the addition
of tamoxifen, 20 mg daily for five years, to the treatment of DCIS
by local excision and radiation therapy decreased the incidence
of invasive cancer recurrence. This benefit was seen independent
of margin status or the presence of comedo-type necrosis. However,
no overall survival benefit was observed over the course of the
study. Whether these observations are justification for the routine
use of tamoxifen in all DCIS patients remains unclear, since the
sub-groups of patients who would benefit the most have not been
defined.
Treatment of recurrence after breast conservation is another crucial
question, since recurrence as invasive cancer is an endpoint that
may affect disease specific survival. If invasive cancer is the
recurrence, its treatment should be as for any other invasive cancer
of the same stage. When DCIS only is the recurrence, a different
question exists. When DCIS only (i.e., without invasive cancer)
occurs after treatment by local excision alone, the usual treatment
is radiation or mastectomy, based upon the same selection criteria
as for DCIS treatment when it occurs for the first time. Therefore,
for women who underwent local excision only, the choices include
re-excision, re-excision and radiation therapy, or mastectomy. For
women who underwent radiation therapy as part of breast conservation
initially, the usual treatment for recurrence, even if the recurrence
was DCIS only, is considered to be mastectomy. There still may be
some patients, depending upon their own preference and understanding
of subsequent risk, who prefer to be treated by local excision alone
in this situation, recognizing that their chance of future recurrence
is higher than they were previously faced with the initial diagnosis
and chose radiation.
As the conference concluded, the panelists unanimously agreed that
the treatment of DCIS is constantly being refined, and the observations
and recommendations made at any time may be influenced by new data
reported almost contemporaneously as well as in the future. Because
this report was based upon the expert opinions and individual experience
of the participants as of the date of the conference, these opinions
must not be construed as dogmatic guidelines for treatment. Management
of individual patients should be based upon each patient’s
unique clinical circumstances. Moreover, the subsequently published
consensus report was not intended to establish specific standards
of care or to be used as a guideline for diagnostic or treatment
management decisions by third-party payers.
Back | Top of Page


|
|



