|
| Risk of Ductal Carcinoma In Situ (DCIS)
Progressing to Invasive Breast Cancer (IBC) D. Craig Allred,
M.D., Professor of Pathology, Breast Center, Baylor College
of Medicine, Houston, TX |
 |
The goals of this presentation are to challenge the widely held
opinions that there are just two subtypes of DCIS (i.e. noncomedo
and comedo) and that comedo DCIS is the most likely to progress
to IBC.
DCIS is the most common (90%) type of noninvasive breast cancer
and is important by giving rise to most IBCs. A practice has developed
over the years to simply dichotomize DCIS into noncomedo and comedo
subtypes based on the notion that the histological features of this
lesion are either well or poorly differentiated, respectively. This
is in contrast to the acknowledged histological variation of IBC
ranging on a continuum from very low to very high grade morphological
abnormalities, and this diversity has been expressed by numeric
grading systems scoring features like growth pattern, nuclear grade,
and mitotic index. In fact, DCIS shows the same wide-ranging histological
diversity as IBC which can also be quantified by similar grading
systems. Recent studies have shown a strong correlation between
the histological and biological differentiation of DCIS along this
continuum. For example, the average rate of tumor cell proliferation
gradually increases from 1% in the lowest grade to over 50% in the
highest grade lesions. Overexpression/amplification of the erbB2
oncogene and mutation of the p53 tumor suppressor gene also increase
in the same direction. In contrast, the expression of estrogen and
progesterone receptors decreases gradually from almost 100% to 25%
from the best to the worst differentiated.
Another commonly held belief is that so-called comedo DCIS is the
most dangerous clinically in the sense of being more likely than
noncomedo DCIS to progress to IBC, and this notion is based on several
types of evidence. For example, comedo DCIS accounted for up to
75% of newly diagnosed lesions in the pre-mammography era and most
were large and palpable. Their largeness was taken to indicate aggressiveness
and their commonness as being likely precursors for most IBC. They
looked bad under the microscope and had aggressive biological features
which correlated with poor prognosis in IBC, so the same was assumed
for DCIS. The rate of axillary nodal metastases was higher with
comedo than noncomedo disease (2-3% vs. <1%). Many studies suggested
that the local recurrence rate of comedo DCIS managed by lumpectomy
was 2-3-fold higher than for noncomedo lesions, especially with
shorter follow-up, and half of all recurrences were invasive.
While these are compelling reasons to think that comedo DCIS has
a particularly bad prognosis, there are reasonable alternative interpretations
for much of this evidence and other equally compelling observations
support the idea that low-grade noncomedo DCIS is MORE likely to
progress to invasive disease. For example, some investigators speculated
that comedo DCIS were common historically because they had trouble
becoming invasive and thus grew large enough to become palpable.
Poorly differentiated histology and aggressive biological features
are clinically meaningless when associated with noninvasive disease.
Because comedo DCIS are often large, it is possible for pathologists
to miss foci of invasion due to routine limited sampling, which
could explain a higher rate of nodal metastases. Then there is other
evidence. For example, at least 80% of IBC have an associated component
of DCIS, and the rate approaches 100% with comprehensive histological
sampling. Surprisingly, the majority (up to 75%) of DCIS in a breast
with IBC are lower-grade noncomedo lesions. The biological correlate
to this is that the 20% rate of erbB2 overexpression in IBC is much
closer to the 10-15% rate associated with noncomedo DCIS than the
60-70% rate observed with comedo disease. In addition, some clinical
studies of DCIS managed by lumpectomy are beginning to show that,
with long follow-up, noncomedo DCIS has a higher rate of recurrence
as IBC.
With mammography, the majority of newly discovered DCIS are now
noncomedo lesions. They are usually smaller and more confined than
comedo lesions, making them easier to manage with conservative surgery.
However, they are probably as likely, if not more so, than high-grade
lesions to progress to IBC if unrecognized or inadequately treated
and, therefore, should be taken very seriously.
| Gray-scale and color Doppler US for local
recurrences of breast cancer Bruno D. Fornage, M.D., Professor
of Radiology & Surgical Oncolory, M. D. Anderson Cancer
Center, University of Texas, Houston, TX |
 |
Because sonography (US) cannot demonstrate microcalcifications
with sufficient reliability, only breast cancer recurrences that
present as a mass can be visualized with US.
Gray-scale Sonographic Appearances
The US appearances of recurrent masses are not significantly different
from those of primary tumors, but the recurrent lesions are usually
smaller at the time of diagnosis. Malignant tumors appear as a focal
hypoechoic mass with irregular or spiculated margins and disruption
of the normal architecture of the breast. In contrast to fibroadenomas
and other benign masses, a recurrent tumor, like a primary carcinoma,
may exhibit a “taller-than-wide” shape, with the tumor’s
longest diameter being perpendicular to the skin (length-to-anteroposterior-diameter
ratio less than 1). This shape is highly characteristic of malignancy.
If the mass is sufficiently large, some heterogeneity of the echotexture
may be noted. Intratumoral clustered microcalcifications can be
visualized as minute bright echoes within the hypoechoic tumor.
Acoustic shadowing-once considered an essential diagnostic feature
of cancer-may be lacking. Absence of compressibility and adherence
of the tumor to the surrounding tissues during dynamic US examination
are very important clues suggesting malignancy.
Scars are difficult to evaluate sonographically because of the
significant shadow associated with them. It is critical to examine
the region of a scar dynamically by increasing the amount of pressure
applied with the probe. This usually clears the shadow that was
cast by a scar, whereas the shadow created by a true recurrent mass
would remained unchanged. Also, scars are retractile and their lateral
edges should be concave, whereas any bulging of the margin of a
scar should be viewed with suspicion. Short-term follow-up is often
needed to confirm the stability of the imaging findings, and US-guided
biopsy may be necessary. In that case, extensive sampling through
the area of shadowing is required.
Invasive lobular carcinomas are difficult to identify on US, as
they are on mammography. This is true for recurrences as well. The
significant distortion and fibrosis seen on mammograms may appear
on sonograms as areas of marked shadowing without a well-defined
mass. Mucinous and medullary carcinomas are relatively well circumscribed.
Medullary cancer may be markedly hypoechoic with significant sound
through-transmission and may mimic a cyst.
Color (Power) Doppler Imaging Findings
Abnormal Doppler signals reflecting hypervascularity have been reported
in the majority of malignant tumors but also in a significant number
of benign masses. In malignant tumors, however, neovessels are typically
tortuous and disorganized and penetrate the tumor at a 90( angle).
With new US techniques like pulse-inversion harmonic imaging and
the use of US contrast agents, detailed mapping of the internal
vascularity of solid masses is becoming possible, which should allow
more reliable differentiation between benign and malignant lesions.
In any case, any new solid mass developing after breast conservation
surgery—especially if hypervascular on color Doppler US—should
be considered suspicious for recurrence until proven otherwise.
Recurrences in Nodal Basins
US can readily visualize recurrences in the nodal basins that are
not amenable to palpation and mammography, such as in the internal
mammary chains and the infraclavicular region.
The confirmation of recurrence, whether in the breast or in the
nodal basins is obtained within minutes through US-guided fine-needle
aspiration.
| The Impact of Local Recurrence After Breast
Conserving Therapy on Distant Metastases and Death Frank A.
Vicini, M.D., Clinical Assoc. Professor, Director of Radiation
Oncology, William Beaumont Hospital, Royal Oak, MI |
 |
Introduction: The impact of local recurrence (LR) on survival in
patients with early stage breast cancer treated with breast conserving
therapy (BCT) remains controversial. Although it has consistently
been demonstrated that patients who experience a LR after BCT have
an increased risk of developing distant metastases (DM), it is uncertain
whether a LR signals a more biologically aggressive tumor or is
the nidus for future dissemination(1;2). For many clinicians, a
LR after BCT is presumed to have no detrimental effect on survival
due to the belief that breast cancer is a systemic disease at inception
(3). As a result, a LR is considered a marker for DM rather than
a cause. In contrast, others believe that preventing a LR may improve
survival by avoiding a “secondary” dissemination of cancer
cells directly from the LR(4). This hypothesis is corroborated by
recent data from three large prospective randomized trials of post-mastectomy
loco-regional radiotherapy (RT) and several published meta-analyses
on the impact of adjuvant RT on survival (5-10).
The purpose of the current analysis was to evaluate the impact
of local recurrence (LR) on the development of distant metastases
(DM), overall survival (OS) and cancer specific survival (CSS) in
patients with early stage breast cancer treated with conservative
surgery (CS) and postoperative radiotherapy (RT) at our institution
and to review published data on this critical issue.
Methods & Materials
Between 1980 and 1995, 1169 patients were treated with CS and RT
at William Beaumont Hospital, Royal Oak, Michigan. All patients
had follow-up greater than one year and (4 nodes involved with cancer.
The median duration of follow-up was 7.7 years. A Cox proportional
hazards model was performed to evaluate the effect of LR on the
development of DM and CSS. A matched pair analysis (MPA) (1:2) was
also performed comparing outcome in patients with and without LR
controlling for multiple prognostic factors.
Results
Local recurrence was 11% at 12 years. For the entire population,
LR led to a poorer OS and CSS at 12 years than local control (LC)
(71% vs 81%, p=0.001 and 69% vs 88%, p<0.001, respectively).
In a Cox multiple regression model, LR was a significant predictor
of cancer specific mortality. The hazard ratio (HR) associated with
LR was 2.69 for mortality and 2.67 for DM (p<0.001 and p<0.001,
respectively). The median time from surgery to distant metastases
was 3.8 years for patients without LR vs 4.7 years for patients
with LR. Patients with LR also had two peaks in the rate of DM (at
2.5 and 6.5 years) as opposed to only one (1.5 years) for those
without LR. The impact of LR on DM was still evident in patients
with small (( 2.0 cm) tumors (p<0.001), negative lymph nodes
(p=0.004) or both (p<0.001). Recurrences developing outside of
the surgical bed region had no negative effect on survival. In the
matched-pair analysis (controlling for age, tumor size, grade, number
of positive nodes, and estrogen receptor status), LR was still the
most significant predictor of mortality (HR 5.86 for mortality and
6.43 for DM).
Conclusions
Our results suggest that LR is responsible for an increase in DM
and cancer-specific mortality in patients treated with CS and RT.
This is reinforced by a distinct difference in the time distribution
of DM after LR and by the fact that recurrences originating outside
of the surgical bed did not affect overall survival. These data
reinforce the necessity to insure optimal LC in patients treated
with BCT and support the conclusions of recent randomized trials
and meta-analyses specifically addressing this issue.
Reference List
- Fisher ER, Anderson S, Tan-Chiu E, Fisher B, Eaton L, Wolmark
N. Fifteen-year prognostic discriminants for invasive breast carcinoma.
Cancer 2001;91:1679-87.
- Fowble B. Ipsilateral breast tumor recurrence following breast-conserving
surgery for early-stage invasive cancer. Acta Oncol 1999;38 Suppl
13:9-17.
- Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola
S, Greco M et al. Local recurrences and distant metastases after
conservative breast cancer treatments: partly independent events.
J.Natl.Cancer Inst. 1995;87:19-27.
- Fortin A, Larochelle M, Laverdiere J, Lavertu S, Tremblay D.
Local failure is responsible for the decrease in survival for
patients with breast cancer treated with conservative surgery
and postoperative radiotherapy. J.Clin.Oncol. 1999;17:101-9.
- Favourable and unfavourable effects on long-term survival of
radiotherapy for early breast cancer: an overview of the randomised
trials. Early Breast Cancer Trialists’ Collaborative Group.
Lancet 2000;355:1757-70.
- Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional
radiation therapy improve survival in breast cancer? A meta-analysis.
J.Clin.Oncol. 2000;18:1220-9.
- Van de SJ, Soete G, Storme G. Adjuvant radiotherapy for breast
cancer significantly improves overall survival: the missing link.
Radiother.Oncol. 2000;55:263-72.
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson
M et al. Postoperative radiotherapy in high-risk postmenopausal
breast-cancer patients given adjuvant tamoxifen: Danish Breast
Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641-8.
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach
F et al. Postoperative radiotherapy in high-risk premenopausal
women with breast cancer who receive adjuvant chemotherapy. Danish
Breast Cancer Cooperative Group 82b Trial. N.Engl.J Med 1997;337:949-55.
- Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco
VE et al. Adjuvant radiotherapy and chemotherapy in node-positive
premenopausal women with breast cancer. N.Engl.J Med 1997;337:956-62.
| Management of Invasive Local Recurrence
in DCIS; Local and Systemic Therapy Patrick I. Borgen, M.D.,
Chief, Breast Service, Department of Surgery, Memorial Sloan-Kettering
Cancer Center, New York, New York |
 |
Ductal carcinoma in situ represents the most rapidly expanding
segment of the breast cancer population in the United States. This
expansion, driven by dramatic improvements in utilization of screening
mammography, has resulted in a significant evolution of the treatment
of ductal carcinoma in situ (DCIS). In broad terms this has paralleled
the evolution of the treatment of invasive carcinoma of the breast.
That is, breast conservation therapy (wide local excision with or
without radiation therapy) is now considered the appropriate and
preferable management option for a majority of patients with localized
DCIS. The role of radiation therapy in DCIS has been addressed in
a number of prospective randomized trials, most notably the NSABP
B17 Trial, which showed not only a substantial reduction in relapses,
but in particular a substantial reduction in invasive relapses.
Along with other studies, this trial changed the paradigm for the
majority of patients with ductal carcinoma in situ, and emphasized
that these patients are at risk for local regional relapse.
There is a wide range of reported local-regional relapse rates
ranging from 5% to nearly 20%. Overwhelmingly the site of recurrence
is at or near the site of previous tumorectomy. (See figure 61-1)
Age is also an important consideration in predicting the likelihood
of local relapse with younger patients experiencing a significantly
higher risk than older patients. Our group has previously shown
that decreased volume of resection in younger patients compared
to older patients may be a contributing factor to this phenomenon.
(See figure below)
One relatively constant figure that has been reported from a majority
of studies is the fact that fully 50% of recurrences are invasive
in nature. Treatment of recurrent invasive disease in the breast
after DCIS can be divided into surgical treatment and systemic treatment.
Both are greatly influenced by the treatment approach taken with
the index DCIS lesion.
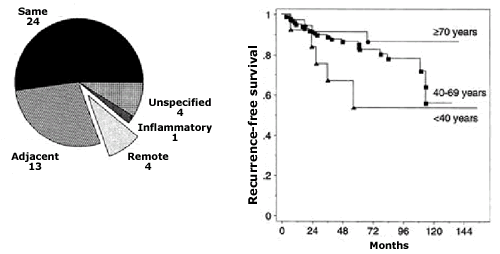
Figure 61-1
Location of local recurrence in relation to the quadrant of the
original carcinoma. (Reproduced by permission from Osborne MP, Borgen
PI, Wong GY, Rosen PP, McCormick B. Savage mastectomy for local
and regional recurrence after breast-conserving operation and radiation
therapy. Surg Gynecol Obstet 1992, 174:189-194. By permission of
Surgery, Gynecology & Obstetrics, now know as the Journal of
the American College of Surgeons.)
Surgical Management of Local Recurrent DCIS
The range of recurrence types after BCT for DCIS ranges from small
microscopic DCIS to inflammatory breast cancer and the surgical
approach depends heavily on this factor. Treatment is also dependent
on the treatment the patient has already received. In the past,
the dogma was to recommend total mastectomy (salvage mastectomy)
for all patients with any form of relapse. This has been called
into question, and it is not unreasonable to, in effect, re-conserve
the breast by performing a wide local excision, particularly if
radiation therapy has not already been administered. It has been
increasingly our practice to offer breast conservation therapy in
these patients. (See algorithm below) For unifocal invasive relapses,
we perform a sentinel lymph node biopsy at the time of the surgical
excision of the primary tumor. There is no evidence that prior surgery
diminishes the success or accuracy of sentinel lymph node biopsy
in breast cancer. More extensive areas of DCIS or multiple disease
areas involving more than one quadrant of the breast are best treated
with mastectomy. Similarly, patients who are previously irradiated,
in the majority of cases, are recommended to undergo a mastectomy
following local relapse in the breast. Approximately 75% of our
patients elect immediate breast reconstruction either with subpectoral
tissue expanders or autogenous tissue transfer. Post mastectomy
radiation therapy is currently done for patients with four or more
positive axillary lymph nodes, involvement of skin, or involvement
of pectoralis major.
Elegant studies in the 1980’s and 90’s indicated that
the overwhelming majority of patients who had invasive cancer initially
and who recurred in the breast were operable because they demonstrated
no evidence of systemic disease. We can extrapolate from this to
recurrences after conservative treatment of DCIS where the majority
of patients will, in fact, have no evidence of systemic disease.

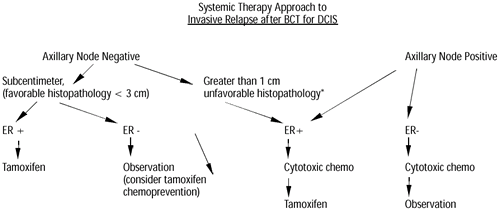
Systemic Therapy Considerations
Systemic therapy options for invasive relapses after conservative
treatment of DCIS very closely parallel management strategies for
patients who present with invasive carcinoma de novo. The prognosis
and treatment are determined primarily by the status of the axillary
lymph nodes and secondarily by tumor characteristics such as size,
lymphovascular invasion, ER/PR status and Her-2/neu status. Patients
who relapse locally in the breast, who are currently on tamoxifen
represent a special therapeutic dilemma, which will be discussed.
There is growing evidence concerning the role of aromatase inhibitors
as anti-estrogen therapy in breast cancer, but specific studies
in DCIS, and in particular recurrent DCIS, have not been reported.
Our understanding of all breast cancer, particularly DCIS is largely
descriptive. Progress in the Human Genome Project holds great promise
for helping us move towards a more functional understanding of the
disease. Approximately 19,000 genes and tens of thousands more tentatively
described as expressed sequence tags (EST) have been identified.
It is likely that many genes that might be useful for diagnosis
andor prognostication in breast cancer have yet to be recognized
in this group. The advent of cDNA microarray technology now allows
for the efficient measurement of expression of virtually every gene
in the human genome. Disease classification and treatment approaches
will evolve as me move towards molecular classification of human
malignancies.
Our current general algorithm for the systemic treatment of invasive
carcinoma of the breast is presented below. Specific therapeutic
agents will be discussed.
References:
- Osborne MP, Borgen PI, Wong GI, et al, Salvage mastectomy for
local and regional recurrence after breast conserving surgery
and radiation therapy. Surg Gyn Obstet 1992; 174:189-194
- Van Zee K, Borgen PI, Memorial Sloan-Kettering Cancer Center
experience, in Ductal Carcinoma In-Situ, Silverstein eds. 1997,
455-467
- Van Zee K, Liberman LL, McCormick B, et al, Long-term follow-up
of DCIS treated with breast conservation : effect of age. Cancer
1999;86(9);1757-1767
- Fisher B, Costantino J, Redmond C, et al, Lumpectomy compared
to lumpectomy with radiation therapy for the treatment of intraductal
breast cancer. N Engl J Med 1993; 328:1581-1586
- Lagios M, Dcut carcinoma in-situ: pathology and treatment.
Surg Clin N Am 1990;70:853-871
- Swallow CJ, Van Zee K, Sacchini V, Borgen PI, Ductal carcinoma
in situ of the breast: progress and controversy. Current Problems
in Surgery 1996; 33(7)553-600
- Hudis CA, Borgen PI, Systemic treatment for stage I and stage
II breast cancer. In BoslGJ and Brennan M (eds) Surgical Oncology
Clinics of North America, Adjuvant Therapy of Cancer. 1997;6(4):683-698
- Van Zee K, Tan LK, Calvano JE, Rosen PP, Borgen PI, p53 mutations
and HER-2/neu amplification in microdissected ductal carcinoma
in situ.
- Alizadeh A, Ross T, Perou C, Towards a novel classification
of human malignancies based upon gene expression patterns. Journal
of Pathology 2001;195:41-52
| Treatment of Axillary Recurrence Gordon
F. Schwartz, M.D., MBA, Professor of Surgery, Jefferson Medical
College, Philadelphia, PA |
 |
Axillary recurrence following appropriately planned and executed
procedures to treat carcinoma of the breast are infrequent. Whether
axillary recurrence is newly recognized cancer in axillary lymph
nodes intentionally or unintentionally left behind at the time of
axillary dissection accompanying breast conservation or mastectomy,
or in the soft tissues of the axilla as recurrence or metastasis,
may significantly affect choice and efficacy of treatment.
As treatment of axillary nodes has changed in response to the “staging
versus treatment” argument about the value of axillary dissection,
with less rather than more extensive dissection of the axilla performed
currently rather than a generation ago, the expectation that a greater
incidence of axillary recurrence would be observed has not been
validated. This does not negate the importance of a meticulous dissection
when treatment of the axilla is indicated.
However, axillary recurrence does occur; when it does it is difficult
to treat. Documenting the nature of the recurrence may be crucial
in determining therapy. Most often, axillary recurrence is noticed
by the patient as a mass of fullness in the axilla. Even more rarely
is recurrence in a Rotter’s (interpectoral) node, which would
present as a fullness in the infraclaviucular space behind or adjacent
to the edge of the pectoralis major muscle. Occasionally, pain,
decreased motion, or even newly noted arm edema may be signs of
axillary recurrence. In general, axillary recurrence is observed
most commonly in the first three years after initial treatment.
Because axillary recurrence may be the first sign of systemic disease,
the diagnosis of axillary recurrence should be made by FNA whenever
possible, so that a complete patient evaluation is performed before
any surgical treatment is planned. When axillary recurrence is accompanied
by supraclavicular node disease, systemic metastasis is almost always
present. CT scan or MRI of the axillary and supraclavicular areas
may help document patient status as well as outline the extent of
disease if a surgical procedure is contemplated.
In the absence of systemic disease, when only a single site (axilla)
can be implicated, a combined surgical and radiation therapy approach
is often appropriate, especially if the disease can be documented
as nodal rather than soft tissue disease. The usual scenario for
this diagnosis is a patient with an inadequate node dissection during
mastectomy or as part of breast conservation. When a node dissection
had not been part of the original treatment plan, and secondary
nodal disease is the diagnosis, a formal node dissection alone may
be appropriate. If a surgical procedure had been performed and the
recurrence is in nodes left behind, a meticulous dissection of the
axilla may be enough, but, depending upon the extent of the disease,
axillary (and supraclavicular radiation) may be added. Rarely, the
recurrence is in an interpectoral node; both surgery and radiation
are then appropriate because of the difficulty performing an en
bloc dissection of this area. Adjuvant chemotherapy (or Tamoxifen)
would usually be considered in all of these patients. Residual nodal
disease may be well controlled by a combination of surgery and radiation
therapy, and long survival is often achieved in these patients with
only axillary node disease. Unfortunately, patients with only small-volume
nodal disease are not the rule, so that the majority of patients
with axillary recurrence ultimate succumb to metastatic breast cancer.
If the recurrence is soft tissue rather than nodal, surgery is usually
not very effective in eliminating further recurrence. Margins are
vague, but large volume disease may be controlled locally, at least
for some time, so that the combination of debulking surgery followed
by radiation therapy, and adjuvant chemotherapy, are the usual recommendations.
Removing “all” of the axillary disease surgically may
be impossible, even macroscopically, but the debulking surgery and
radiation may offer major improvement in the quality of patients’
lives, since chest wall and axillary recurrence become a source
of extreme discomfort and embarrassment because of difficulty maintaining
hygiene as the disease progresses.
An emerging problem in the next ten years may be an increase in
the incidence of axillary node recurrence in patients who have undergone
a “negative” sentinel lymph node biopsy. Because this
procedure is still in its relative infancy, without lengthy follow-up
data, the true incidence of false negative sentinel node biopsy
is uncertain. Thus far, with close to ten years experience in the
procedure by some investigators, axillary recurrence has been anecdotal.
Hopefully that will continue as the procedure is more widely adopted
in this country and abroad.
| Should adjuvant therapy be given to all
patients? Daniel F. Hayes, M.D., Director, Breast Oncology Program,
University of Michigan Comprehensive Cancer Center, MI |
 |
Based on predictive factor categories (chemotherapy: age, ? ER,
HER2; endocrine therapy: ER, PgR), the relative benefits from specific
adjuvant systemic therapy (AST) can now be estimated 1,2. For individual
patients, the absolute benefits for individual patients based on
their prognostic (nodal status, tumor size, grade, and to a lesser
extent, ER, PgR, and HER-2) and predictive factor profiles 3. Therefore,
a woman can be informed about her odds of reducing the chance of
developing subsequent, incurable recurrence from specific therapies.
The decision about whether to accept one type of AST or another
is complex. Preliminary studies have suggested that different patients,
caregivers, and societies make decisions differently based on social,
cultural, and economic factors 4,5. Furthermore, the endpoint (reduction
in mortality, metastasis, local-regional recurrence, or new primary)
substantially modifies these decisions. In summary, the answer to
the question is no, but a thoughtful discussion with each patient
will allow her to be the respondent.
- Early Breast Cancer Trialist’s Collaborative Group: Tamoxifen
for early breast cancer: An overview of the randomised trials.
Lancet 351:1451-1467, 1998
- Early Breast Cancer Trialist’s Collaborative Group: Polychemotherapy
for early breast cancer: an overview of the randomized trials.
Lancet 352:930-42, 1998
- Ravdin PM, Siminoff L A, Davis GJ, et al: Computer program to
assist in making decisions about adjuvant therapy for women with
early breast cancer. J Clin Oncol 19:980-91, 2001
- Coates AS, Simes RJ: Patient assessment of adjuvant treatment
in operable breast cancer. New York, NY, John Wiley & Sons
Ltd, 1992
- Lindley C, Vasa S, Sawyer T, et al: Quality of life and preferences
for treatment following systemic adjuvant therapy for early stage
breast cancer. Journal of Clinical Oncology 16:1380-87, 1998
| Overview of clinical studies on liposome
therapy I. Craig Henderson, M.D., Adjunct Professor of Medicine,
University of California, San Francisco, CA |
 |
Liposomal encapsulation of the anthracyclines results in reduced
cardiotoxicity. Two formulations of doxorubicin have been widely
studies, Doxil and Myocet, but only the former has been approved
for marketing in the U.S. The differences between the two formulations
result from the addition of polyethylene glycol or PEG to the outer
surface of Doxil. Although all liposomal formulations have a longer
half life than doxorubicin, the half life of Doxil is particularly
long and exceeds 50 hours. The optimal dose-schedules for the two
drugs is quite different. Myocet is usually administered every 3
weeks at a dose of 75 mg/m2 as a single agent or 60 mg/m2 in combination.
Doxil is usually given every 4 weeks at a dose of 40-50 mg/m2 as
a single agent and 30-35 mg/m2 in combination every 4 weeks. Both
of these drugs have been compared as a single agent in randomized
trials with other anthracyclines. The data are difficult to interpret,
but in general the activity of the liposomal formulations is very
nearly the same as that of doxorubicin or (in the case of Myocet)
epirubicin. Both drugs have substantially and significantly less
cardiotoxicity than doxorubicin. Both drugs have been evaluated
in combinations. Trials of the liposomal drugs plus Herceptin are
underway, but no definitive results are available yet or likely
to be available soon. The toxicity profiles of the two drugs differ
substantially. Except for cardiotoxicity, the side effects and dose
limiting toxicity of Myocet is similar to that of doxorubicin. Doxil
is associated with almost no hair loss or nausea/vomiting; myelosuppression
is reduced compared to doxorubicin. The dose limiting toxicity from
Doxil is palmar plantar erythrodysesthesia, but this can be substantially
reduced by using lower doses with longer intervals. This class of
drugs holds considerable promise for less toxic regimens for the
management of breast cancer, especially for those who are particularly
adverse to toxicities, for those who are at higher than average
risk for cardiotoxicity, and in combination with drugs that synergize
with the anthracyclines in inducing cardiotoxicity.
| Her2 Determinations In Decision Making For
the Treatment of Breast Cancer Peter M. Ravdin, Associate Professor
of Medical Oncology, University of Texas, Health Science Center,
San Antonio, TX |
 |
This talk will review this rapidly evolving area of interest. The
talk will show how the ASCO guidelines for the use of Her2 might
be challenged and review each of the 7 ASCO guideline statements
with an eye as to whether these guidelines might be revised again
in the near future. Admittedly one of the areas in the ASCO tumor
guidelines that have gotten more complex and evolved over successive
iterations is the topic of the use of Her2 as a tumor marker. The
most recent ASCO guidelines suggest:
1. That Her2 expression level should be measured on every primary
breast cancer, and suggested that measures of Her2 amplification
might also be of value.
Is there any role for using Her2 by IHC? Examination of the role
of FISH testing is now emerging as a more useful test and that measures
of expression are being eclipsed. But need Her2 be determined prior
to needing to know this information at the time of recurrence?
2. It was recommended that Her2 overexpression be used to identify
patients most likely to benefit from Trastuzumab (Herceptin) treatment
of metastatic, recurrent, or treatment refractory breast cancer
(but not yet in adjuvant therapy). FISH measures of amplification
are certainly more predictive.
3. It was recommended that Her2 determinations not be used determine
whether CMF was appropriate adjuvant therapy. This seems to be holding
up and is a good example how underpowered studies with subset analysis
can be misleading.
4. It was felt that Her2 overexpression might identify patients
who would particularly benefit from anthracycline based adjuvant
therapy, but that the lack of Her2 overexpression should not be
used to select against anthracycline based adjuvant therapy.
A curiously hedged statement. The data will be reviewed and be
found to be provocative but still not definitive in support of the
use of predictive power of Her2 determination to select for or against
the use of anthracyclines in adjuvant therapy.
5. It was recommended that Her2 determinations not be used to determine
whether endocrine therapy was appropriate in adjuvant therapy or
the treatment of metastatic disease.
Although this seems to be true, Her2 positive patients do seem
to benefit less from tamoxifen less than Her2 negative patients
even in studies where all patients were estrogen receptor positive.
Interesting preliminary studies suggest that Her2 positive patient
tumors do not have this relative resistance to aromatase inhibitors.
If true then Her2 might be used to select patients who particularly
benefit from aromatase inhibitors.
6. It was recommended that Her2 determinations not be used determine
whether the use of taxanes was appropriate in adjuvant therapy or
the treatment of metastatic disease.
This seems to be holding up because this question has not been
adequately tested.
It was felt that the data was insufficient to recommend the use
of Her2 overexpression to identify patients with a higher risk of
relapse.
An area of strong controversy particularly for overexpression.
Interestingly a review of the current literature suggests that Her2
amplification may be a better test with greater prognostic import.
1. 2000 Update of Recommendations for the Use of Tumor Markers
in Breast and Colorectal Cancer: Clinical Practice Guidelines of
the American Society of Clinical Oncology. Robert C. Bast, Jr, Peter
Ravdin, Daniel F. Hayes, Susan Bates, Herbert Fritsche, Jr, John
M. Jessup, Nancy Kemeny, Gershon Y. Locker, Robert G. Mennel, and
Mark R. Somerfield JCO Mar 15 2001: 1865-1878.
| Optimal Chemotherapeutic Regimens: Duration,
Timing, and Schedule Clifford Hudis, M.D., Chief, Medical Oncology
of Breast Center, Memorial Sloan-Kettering Cancer Center, New
York City, NY |
 |
The most advantageous means of timing and sequencing surgery, radiation
therapy, and systemic therapy is uncertain and is the subject of
ongoing clinical trials. Because chemotherapy successfully shrinks
locally advanced breast cancers and can allow local control surgery
in the majority of cases, there has been enthusiasm for broader
use of neo-adjuvant therapy. Neo-adjuvant therapy has been shown
to allow more frequent breast conservation in patients with initially
resectable disease but this earlier use of chemotherapy per se does
not influence the risk of relapse or death, although it may provide
early information on prognosis. Based on this data, neo-adjuvant
chemotherapy can be most easily defended for patients with initially
unresectable breast cancer and for those who refuse to undergo mastectomy
but for whom a limited excision and radiation therapy would be acceptable.
On the other hand, it is possible that the risks of over treatment
for low risk patients (i.e. chemotherapy given for largely in situ
carcinomas) could outweigh the benefits of this approach.
The optimal duration and make-up of chemotherapy, whether given
post-operatively or in the neo-adjuvant setting is not known. Six
months of CMF is a gold standard based on the worldwide meta-analysis
performed at Oxford University but a variety of other regimens could
be superior or equally effective and less toxic. Three months of
AC was equivalent to CMF in two NSABP trials while six months of
CAF or CEF has been superior in most trials. How six months of therapy
consisting of sequentially dosed AC and a taxane will fit in remains
to be confirmed. Numerous clinical trials are exploring these issues
and will be reviewed.
| New Trends In Adjuvant/Neoadjuvant Chemotherapy
Terry Mamounas, M.D., M.P.H., F.A.C.S., Associate Professor
of Surgery, Northeastern Ohio Universities College of Medicine
, Medical Director, Aultman Cancer Center, Canton, OH |
 |
The benefit from adjuvant chemotherapy has been convincingly demonstrated
in patients with stage I and II breast cancer. In these patients
adjuvant chemotherapy has been shown to substantially reduce the
risk for recurrence and death.1 However, despite significant progress,
several issues regarding adjuvant/neoadjuvant chemotherapy still
remain outstanding2 and these will be the focus of the presentation.
Optimal Anthracycline-Containing Regimens
The 1995 Oxford Overview analysis demonstrated that, when compared
to CMF alone, anthracycline-containing regimens produce somewhat
greater reduction in recurrence and mortality.1 Randomized trials
have shown a threshold effect for doxorubicin and cyclophosphamide,
in the AC combination with no additional benefit seen with doses
over 60mg/m2 of doxorubicin and 600mg/m2 of cyclophosphamide.3-5
However, there is still uncertainty as to which anthracycline-containing
regimen is optimal. Randomized trials by the NSABP6, 7 have shown
equivalence in efficacy between six cycles of the conventional CMF
regimen and four cycles of AC. On the other hand, results of a U.S.
Intergroup (INT 0102) trial8 and an NCIC trial9 have demonstrated
statistically significant improvement in disease-free survival and
overall survival with six cycles of CAF or six cycles of CEF when
compared to six cycles of CMF in node-negative and node-positive
breast cancer patients respectively. In both these trials the observed
proportional reduction in mortality with the use of the anthracycline-containing
regimen was in the range of 20-25%. The proportional reduction in
mortality with anthracycline-containing versus non anthracycline-containing
regimens observed in the 1995 overview was 11% but this analysis
included various anthracycline-containing regimens (AC, EC FAC,
FEC, etc.) given for varying number of cycles (4 to 12). Although
it is uncertain whether some anthracycline-containing regimens are
more active than others, the data indirectly suggest that six cycles
of CAF or CEF might be more active than 4 cycles of AC. Proposed
adjuvant trials by the U.S. cooperative groups will attempt to definitively
address some of these questions.
Role of Taxanes as Adjuvant/Neoadjuvant Therapy
The role of taxanes in the adjuvant setting and the optimal way
of integrating them with the other chemotherapy agents is still
controversial and evolving. So far, two large adjuvant trials in
node-positive patients (CALBG 9344 and NSABP B-28) and one large
neoadjuvant trial (NSABP B-27) in patients with operable breast
cancer have produced results using the sequential administration
of paclitaxel or docetaxel following AC. Mature results from the
first adjuvant trial show a small but statistically significant
improvement in disease-free survival (13% reduction in the odds
of recurrence) and a small, not statistically significant improvement
in overall survival (14% reduction in the odds of death) with the
addition of paclitaxel to AC.10 Most of the benefit was seen in
receptor negative patients (25% reduction in the odds of recurrence
and 22% reduction in the odds of death) although this subset analysis
was unplanned. Preliminary results from the second trial (with 34
months of median follow up) do not show a benefit in disease-free
or overall survival with the addition of paclitaxel to AC.11 However,
a similar but non-significant trend to that seen in CALGB 9344 in
favor of adding paclitaxel was seen patients that did not receive
tamoxifen (most likely ER-negative). The NSABP B-27 trial compared
neoadjuvant or adjuvant docetaxel following neoadjuvant AC to neoadjuvant
AC alone. The addition of neoadjuvant docetaxel significantly increased
clinical complete response rates (from 40% to 65%), pathologic complete
response rates (from 13.7% to 25.6%) and the percentage of patients
with histologically negative axillary nodes (51.5% vs. 59.5%)12
indicating additional antitumor activity with sequential docetaxel
following AC. However, outcome results from this trial are not available
yet. Two ancillary studies to the B-27 trial are exploring the potential
relationship between serum/tumor biomarkers and response to preoperative
AC and/or docetaxel chemotherapy and outcome.
One issue that has emerged regarding the sequential anthracycline-taxane
trials is whether the observed benefit might be the result of administration
of additional cycles of chemotherapy in the experimental group (4
vs 8 cycles) and not necessarily the result of administration of
non-cross resistant chemotherapy. Two trials have attempted to address
this issue. In the first randomized trial from the M.D. Anderson
Cancer Center the role of paclitaxel was evaluated in the neoadjuvant/adjuvant
setting. This trial compared four cycles of preoperative/post-operative
paclitaxel to four cycles of preoperative/postoperative FAC. In
both groups, four additional cycles of adjuvant FAC were given postoperatively.
Thus, in terms of DFS and overall survival, this study effectively
compared eight cycles of FAC with four cycles of paclitaxel followed
by four cycles of FAC, testing whether the sequential administration
of two clinically non-cross resistant regimens (given for four cycles
each) might be more advantageous than the administration of one
of the regimens given for the same total number of cycles (eight
cycles of FAC). Results from the neoadjuvant part of that trial
on 174 patients,13 demonstrated a similar rate of overall clinical
response and similar extent of residual disease at the time of surgery
between the two treatment groups. Preliminary outcome results for
524 patients were recently disclosed14 and demonstrated that the
4-year DFS was 85% for paclitaxel compared with 81% for FAC (p=0.2).
Although there was a trend toward a better outcome in patients who
received the noncross-resistant regimens (paclitaxel X 4 followed
by FAC X 4) compared with those who received FAC X 8, the difference
was not statistically significant.
This concept was also tested by a somewhat different approach in
a smaller randomized trial conducted in Aberdeen, UK.15,16 In this
trial, patients with large operable ((4 cm) or locally advanced
(T3-4, TxN2) breast cancer were given four cycles of preoperative
cyclophos-phamide, vincristine, doxorubicin, prednisolone (CVAP)
and, if they responded, were randomly assigned to receive four more
cycles of pre-operative CVAP or four cycles of preoperative docetaxel.
Patients who did not respond were given four cycles of docetaxel.
After comple-tion of chemotherapy, final tumor response was assessed
and appropriate surgery, which included assessment of pathologic
response, was performed. Of 167 patients who were given initial
chemotherapy with CVAP, 102 (61%) had a clinical response and were
judged to be suit-able for randomization. Median follow up was 38
months. Those who continued on four more cycles of CVAP had a final
clinical response rate of 66%, whereas those who were given docetaxel
had a significantly higher final clinical response rate of 94%.
More importantly, com-plete pathologic response in the randomized
patients was 18% with CVAP X 8 and significantly higher (34%) with
CVAP X 4/ docetaxel X 4. This difference in pathologic response
rates translated to a survival improvement. In patients randomized
to receive further CVAP, the 3-year survival was 84%, while in those
randomized to docetaxel, 3-year survival was 97% (p=0.02; log-rank
test). In patients randomized to receive further CVAP, the 3-year
disease-free interval was 77% while in those randomized to receive
docetaxel, the 3-year disease-free interval was90% (p=0.03; log-rank
test).
Finally, combinations of anthracyclines and taxanes have been found
to be very active in phase II-III trials in patients with advanced
breast cancer and several adjuvant trials have compared or are in
the process of comparing doxorubicin-taxane combinations (with or
without cyclophosphamide) to AC, FAC or AC followed by taxane. These
trials will, hopefully, shed light on the optimal way of integrating
taxanes into the adjuvant setting.
References
- Early Breast Cancer Trialists’ Collaborative Group: Polychemotherapy
for early breast cancer: an overview of the randomized trials.
Lancet 1998; 352:930-42.
- 2. 2000 NIH Consensus Development Conference on Adjuvant Breast
Cancer Treatment: November 1-3, 2000, Bethesda, MD.
- Fisher B, Anderson S, Wickerham DL, et al: Increased Intensification
and Total Dose of Cyclophosphamide in a Doxorubicin-Cyclophosphamide
Regimen for the Treatment of Primary Breast Cancer: Findings from
National Surgical Adjuvant Breast and Bowel Project B-22. J Clin
Oncol 1997; 15:1858-69.
- Fisher B, Anderson S, DeCillis A, et al: Further Evaluation
of Intensified and Increased Total Dose of Cyclophosphamide for
the Treatment of Primary Breast Cancer: Findings from National
Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol
1999; 17:3374-88.
- Henderson IC, Berry D, Demetri C, et al.: Improved disease
free survival (DFS) and overall survival (OS) from the addition
of sequential paclitaxel (T), but not from the escalation of doxorubicin
(A) dose level in the adjuvant chemotherapy of patients (PTS)
with node-positive primary breast cancer (BC). Proc Am Soc Clin
Oncol 1998; 17:101a, abstract.
- Fisher B, Anderson S, Tan-Chiu E, et al: Tamoxifen and Chemotherapy
for Axillary Node Negative, Estrogen receptor-Negative Breast
Cancer: Findings from the National Surgical Breast and Bowel Project
B-23. J Clin Oncol 2001; 19:931-42.
- Fisher B, Brown A, Dimitrov N, et al: Two Months of Doxorubicin-Cyclophosphamide
With or Without Interval Reinduction Therapy Compared with Six
Months of Cyclophosphamide, Methotrexate, and Fluorouracil in
Positive-Node Breast Cancer Patients With Tamoxifen Nonresponsive
Tumors: Results from the National Surgical Adjuvant Breast and
Bowel Project B-15. J Clin Oncol 1990; 8:1483-96.
- Hutchins L, Green S, Ravdin P, et al: CMF versus CAF with and
without tamoxifen in high-risk node-negative breast cancer patients
and a natural history follow up study in low-risk node-negative
patients: First results of Intergroup Trial 0102. Proc Am Soc
Clin Oncol 1998; 17:1a, abstract.
- Levine MN, Bramwell VH, Pritchard KI, et al: Randomized Trial
of Intensive Cyclophosphamide, Epirubicin, and Fluorouracil Chemotherapy
Compared With Cyclophosphamide, Methotrexate, and Fluorouracil
in Premenopausal Women With Node-Positive Breast Cancer. J Clin
Oncol 1998; 16:2651-58.
- Henderson IC: 2000 NIH Consensus Development Conference on
Adjuvant Breast Cancer Treatment: November 1-3, 2000, Bethesda,
MD.
- Mamounas EP: 2000 NIH Consensus Development Conference on Adjuvant
Breast Cancer Treatment: November 1-3, 2000, Bethesda, MD.
- Bear H: The effect on primary tumor response of adding sequential
Taxotere to Adriamycin and cyclophosphamide: preliminary results
from NSABP protocol B-27. Breast Cancer Res Treat 2001; 69:210,
Abstract 5.
- Buzdar AU, Singletary SE, Theriault RL, et al: Prospective
evaluation of paclitaxel versus combination chemotherapy with
fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant
therapy in patients with operable breast cancer. J Clin Oncol
1999; 17:3412-17.
- Thomas E, Buzdar A, Theriault R, et al: Role of paclitaxel
in adjuvant therapy of operable breast cancer: preliminary results
of prospective randomized clinical trial. Proc Am Soc Clin Oncol
2000; 19:74a, abstract.
- Hutcheon AW, Ogston KN, Heys SD, et al: Primary chemotherapy
in the treatment of breast cancer: significantly enhanced clinical
and pathological response with docetaxel. Proc Am Soc Clin Oncol
2000; 19:83a, abstract.
- Hutcheon AW, Heys SD, Miller ID, et al: Improvements in survival
in patients receiving primary chemotherapy with docetaxel for
breast cancer: a randomised controlled trial. Breast Cancer Res
Treat 2001; 69:298, Abstract 506.
| New molecular diagnostics to aid in choosing
therapy Debu Tripathy, M.D., UCSF Carol Franc Buck Breast Care
Center, University of California, San Francisco, CA |
 |
The benefits of system therapy for breast cancer are typically
defined through clinical trials with the broadest possible eligibility
criteria. Therefore, the benefits such as response rate and time
to disease progression for metastatic disease, or disease-free and
overall survival for early stage disease represent population averages.
Only in the case of hormonal therapy and HER2/neu-targeted therapy
is there an estab-lished basis for tissue testing in order to choose
therapy. However, retrospective analyses of large clinical trials
have in some cases
suggested that specific host and tumor tissue markers might influence
outcome in a treatment-specific fashion. These analyses are confounded
by several facts:
* Subsets of interest are often small and hence the statistical
power is limited and not definitive
* Assays and interpretation for specific protein or genetic markers
have not been standardized and this makes it difficult to compare
or combine studies
* Some marker may behave as prognostic markers in that they predict
outome independent of therapy or are predictive in that they only
predict a differential outcome in regards to a specific therapy.
Many markers actually have both prognostic and predictive properties
The following markers are either established or under study for
predictive or prognostic value
| Prognostic |
Predictive |
Predictive and Prognostic |
| Nodal status |
Age (chemotherapy) |
Tumor grade/proliferative index |
| Tumor size |
ER/PR (hormonal therapy) |
HER2/neu |
Disease free interval
|
|
|
Stage/tumor burden
|
|
|
| Functional status |
|
|
Predictive markers have the potential to aid in choosing optimal
therapy not only to maximize the benefit but to spare toxicity to
those not likely to have a therapeutic response. The obvious example
is the well documented lack of benefit of hormonal therapy such
as tamoxifen and aromatase inhibitors in patients whose tumors are
negative for both estrogen and progesterone receptors. There is
now growing evidence that a certain threshold of HER2/neu expression
as defined by either immunohistochemical score or gene amplification
predicts response to trastuzumab (Herceptin) such that those below
the cutoff should both receive this drug and avoid the associated
cardiotoxicity risk.
New molecular and protein diagnostics that predict responses to
specific therapies are being pursued intensively although none are
ready for routine use. In general, predictive markers must have
certain properties, the extent of which will determine their clinical
utilite. These include:
* Discriminatory power (odds of response or disease free/overall
survival associated with presence of marker)
* Prevalence of the marker
* Reprodicibility and feasibility of assay
* Well defined cutpoint to define positivity of markers
Components of signaling pathways that are related to the drug of
interest or that deal with the metabolism or cell transport of the
drug are candidate markers for study. Specific markers being assessed
include the following. Note that some studies are attempting to
assess tumor tissue either at baseline or after therapy while others
are assessing host factors, such as such as drug metablism or membrane
transporters, that would affect the anti-tumor activity of the drug
of interest
| Tissue Markers |
Tissue Marker pre/post Therapy |
Host Markers |
| Thymidylate synthetase |
Proliferative indices
(eg. Ki-67) |
Cytochrome P450 enzyme family polymorphisms |
| MDR and other transporter proteins |
Bcl-2 and other apoptosis-associated proteins |
Cell membrane transporter (efflux)
polymorphisms |
| Microvessel density, other markers of angiogenesis (VEGF and
VEGF receptors) |
Stress response proteins |
DNA repair enzyme polymophisms |
| Proteases, protease inhibitors, integrins |
Cyclins, cyclin dependent kinases (CKI)
and CKI inhibitors |
|
Signal transduction proteins
(eg.c ras, erk, akt, PI3K) |
DNA repair enzymes |
|
This list represents general classes of genes and proteins. No
specific marker has yet been validated prospectively. New technology
that allows high throughput analysis of a broad array of genes or
proteins has facilitated the development of specific genetic prognostic
and predictive markers. In animal tumor xenograft studies, discreet
genes are upregulated and downregulated differentially based on
responsiveness to specific chemotherapeutic agents. However, the
statistical complexity of analyzing very large number of markers
requires that some selectivity be applied. For example, rather than
analyzing 20 to 30 thousand genes, it is more practical to focus
on genes sets of functional relevance such as those outlined on
the table above. Statistical tools such as hierarchical clustering
can identify groups of genes that are associated with a particular
phenotype such as responsiveness to specific therapy. Thus, even
without knowing the function of the genes in such a “cluster”,
specific profiles might be defined that would predict sensitivity
or resistance to therapy, particularly therapy that targets a specific
biological pathway.
Most large cooperative group trials now have an extensive effort
to collect tumor tissue blocks. Retrospective analyses for predictive
markers that are generated from smaller pilot studies can be validated
with these resources. Ultimately, prospective trials that determine
therapy on the basis of predictive markers will need to be done
to determine the true value of any marker. As cancer treatments
evolve to biologically targeted therapies, this will be even more
critical.
| Assessing Hormone Receptors: How Accurate
Are Our Measurements? D. Craig Allred, M.D., Professor of Pathology,
Breast Center, Baylor College of Medicine, Houston, TX |
 |
Estrogen receptors (ER) and progesterone receptors (PR) are the
most important biomarkers in breast cancer. Determining their status
is essential in deciding how to treat all patients with breast cancer.
Until about 5 years ago, standardized biochemical ligand binding
assays (LBAs) were used to assess ER and PR in nearly all laboratories.
Over the past 5 years, however, the LBAs have been essentially replaced
by immunohistochemistry (IHC) on formalin-fixed paraffin-embedded
tumor tissue. Is this good?
There are several economical, logistical, and technical advantages
to using IHC over the LBA, which is why most laboratories changed
over. Like any procedure, however, IHC must be performed properly
to obtain accurate results. Unfortunately, because of the diverse
and often sub-optimal methodologies being used in laboratories around
the world, up to 20% of the IHC results are probably inaccurate
(1-4), which is unacceptable. Most of the inaccuracies are false-negatives,
meaning that each year perhaps 40,000 newly diagnosed breast cancer
patients are being denied the potential benefits of hormonal therapy
in this country alone.
The College American Pathologists (CAP) recently approved the use
of IHC for assessing ER and PR in routine clinical practice without
providing specific guidelines as to how the tests should be performed
(5). However, “expert” panels of pathologists, oncologists,
and surgeons have published general guidelines for assessing and
judging the worth of tumor biomarkers (5-8). These guidelines all
agree that markers used in routine practice should be clinically
validated, technically validated, and useful. Clinical validation
means that the test being used to measure the marker identifies
groups of patients with significantly different risks of relapse,
survival, or treatment response that ideally have been demonstrated
in multiple randomized studies. Technical validation means that
the test is specific, sensitive, reproducible, calibrated to patient
outcome, and interpreted in a relatively uniform manner from laboratory
to laboratory. Useful means that the results are actually used by
physicians to make treatment decisions.
It is difficult to know exactly what methodologies are being used
in the thousands of laboratories performing IHC tests for ER and
PR. If the peer-reviewed medical literature is any indication, then
methods and quality vary enormously, and it is quite likely that
problems in the real world on a daily basis are much larger than
indicated by scientific publications which, themselves, are problematic.
There are about 50 published studies assessing the relationship
between ER status by IHC and patient response to hormonal therapy
in one setting or another (9, 10). The design and quality of these
studies vary considerably, but nearly all reported a significant
correlation between a positive test (i.e. detectable ER expression
in tumors) and favorable response to hormonal therapy, which is
encouraging and suggests that clinical validation for ER by IHC
is close if not already achieved. From a technological point of
view, however, these studies used diverse and largely obsolete methodologies
such as relatively insensitive antibodies on frozen tissue sections.
Such methods are nearly useless in today’s laboratories where
testing is virtually restricted to formalin-fixed tissue and newer
more sensitive antibodies with little or no scientific track record.
A handful of recent studies have been published which go a long
way towards validating some of the newer reagents and methodologies
(9, 11-14). If adopted widely, which has not happened, overall accuracy
and reproducibility of ER testing would improve dramatically.
The problems with testing for PR by IHC are much larger than for
ER. Far fewer clinical studies have been published, their results
have been mixed, and their methodologies diverse and based almost
exclusively on frozen tissue and older antibodies that are no longer
available (9, 10). To date, no substantial studies have been published
validating the performance of newer PR antibodies in fixed-archival
tissue, so there are few if any methodologies for laboratories to
emulate.
On a national and global scale, when measured against the scientific
principles and guidelines for assessing tumor biomarkers that have
been published (refs), the clinical and technical validation of
IHC tests for ER and PR have not been achieved, yet they are being
performed daily in thousands of laboratories and oncologists are
using the information to treat patients with breast cancer. It is
a near certainty that a significant proportion (up to 20%) of patients
are being mistreated because of inaccurate results (usually false
negatives), which will contin-ue until methodologies are adequately
validated and implemented on a large scale. There are many unequal
reagents and methodologies for laboratories to chose from and their
choices currently appear to be influenced more by vendor advertising
and cost than scientific validity.
The CAP currently has plans to implement educational programs for
laboratories to help alleviate some of these problems. Until then,
laboratories performing these tests should describe their methods
in some detail (especially naming the antibodies being used), report
results explicitly (% positive cells, etc.), and interpret results
as “positive” or “negative” only if they have
been calibrated to clinical outcome. Ideally they should adopt published
validated methodologies that can be referenced in their reports.
Oncologists using these tests in treating patients should be wary
of “negative” results unless the laboratories they rely
on use validated methodology and, if not, should probably have them
repeated in laboratories that do.
References
- Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor
status by immunohistochemistry is superior to the ligand-binding
assay for predicting response to adjuvant endocrine therapy in
breast cancer. J Clin Oncol 1999;17:1474-1481.
- Rhodes A, Jasani B, Barnes D, Bobrow L, Miller K. Reliability
of immunohistochemical demonstration of oestrogen receptors in
routine practice: interlaboratory variance in the sensitivity
of detection and evaluation of scoring systems. J Clin Pathol
2000;53:125-130.
- Rhodes A, Jasani B, Balaton A, Miller K. Immunohistochemical
demonstration of oestrogen and progesterone receptors: correlation
of standards achieved on in house tumours with that achieved on
external quality assessment material in over 150 laboratories
from 26 countries. J Clin Pathol 2000;53:292-301.
- Rhodes A, Jasani B, Balaton A, Barnes D, Anderson E, Bobrow
L, et al. Study of interlaboratory reliability and reproducibility
of estrogen and progesterone receptor assays in Europe. Am J Clin
Pathol 2001;115:44-58.
- Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark
GC, et al. Prognostic factors in breast cancer. College of American
Pathologists consensus statement 1999. Arch Pathol Lab Med 2000;124:966-978.
- McGuire WL. Breast cancer prognostic factors: Evaluation guidelines.
J Natl Cancer Inst 1991;83:1-9.
- Hayes DF, Bast RC, Desch CE, Fritsche H, Kemeny NE, Jessup
JM, et al. Tumor marker utility grading system: a framework to
evaluate clinical utility of tumor markers. J Natl Cancer Inst
1996;88:1456-1466.
- Panel AE. 1997 update of recommendations for the use of tumor
markers in breast and colorectal cancer. J Clin Oncol 1998;16:793-795.
- Allred DC, Harvey JM, Berardo MD, Clark GC. Prognostic and
predictive factors in breast cancer by immunohistochemical analysis
(Review). Mod Pathol 1998;11(2):155-168.
- . Mohsin SK, Allred DC. Immunohistochemical biomarkers in breast
cancer (Review). The J Histotechnol 1999;22:249-261.
- . Alberts SR, Ingle JN, Roche PR, Cha SS, Wold LE, Farr GH,
et al. Comparison of estrogen receptor determinations by a biochemical
ligand-binding assay and immunohistochemical staining with monoclonal
antibody ER1D5 in females with lymph node positive breast carcinoma
entered on two prospective clinical trials. Cancer 1996;78:764-762.
- Barnes DM, Harris WH, Smith P, Millis RR, Rubens RD. Immunohistochemical
determination of oestrogen receptor: comparison of different methods
of assessment of staining and correlation with clinical outcome
of breast cancer patients. Br J Cancer 1996;74:1445-1451.
- Clahsen PC, van de Velde CJH, Duval C, Pallud C, Mandard AM,
Delobelle-Deroide A, et al. The utility of mitotic index, oestrogen
receptor and Ki-67 measurements in the creation of novel prognostic
indices for node-negative breast cancer. Eur J Surgical Oncol
1999;25:356-363.
- Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, et
al. Estrogen receptor (ER) and progesterone receptor (PgR) by
ligand-binding assay compared with ER, PgR, and pS2 by immunohistochemistry
in predicting response to tamoxifen in metastatic breast cancer:
A Southwest Oncology Group Study. Int J Cancer 2000;89:111-117.
| Estrogen receptor function: The laboratory
and clinical data on Faslodex. C. Kent Osborne, M.D., Professor
of Medicine & Cell Biology, Baylor College of Medicine,
Houston, TX |
 |
Estrogen receptor (ER) is an important diagnostic and treatment
target in breast cancer. ER is activated by ligand-binding and undergoes
a confirmational change that permits association with coregulatory
proteins. Phosphorylation of ER by ligand-binding or through the
action of MAP kinases also influences receptor function. The receptor
can then influence gene expression by both classical and non-classical
mechanisms. There is some evidence that ER can also act on the membrane
to stimulate growth factor pathways. Coactivator proteins are important
for ER function and an abundance of coactivators can increase the
estrogen agonist activity of drugs such as tamoxifen, which have
a mixed activity spectrum. Tamoxifen-stimulated growth, due to its
increasing agonist effects, is one mechanism for tamoxifen resistance
in patients. This type of resistance generated great interest in
pure antiestrogens that have no estrogen agonist qualities. Fulvestrant
(Faslodex) is a steroidal antiestrogen with pure antagonist qualities.
It binds to the estrogen receptor with high affinity and blocks
gene expression more completely than tamoxifen. In preclinical models
Faslodex was a more potent anti-tumor agent than tamoxifen or estrogen
withdrawal, and Faslodex was shown to inhibit tamoxifen-stimulated
tumors. This activity of Faslodex in tamoxifen-resistant patients
was confirmed in a Phase II trial, and subsequent Phase III trials
demonstrate that it is at least as effective as aromatase inhibitors
in patients who are resistant to tamoxifen. Faslodex represents
a new class of endocrine agents that should help clinicians and
patients in the management of hormone responsive breast cancer.
| Mechanisms of resistance to endocrine therapy
Professor Anthony Howell, CRC Department of Medical Oncology,
University of Manchester, UK |
 |
Increases in our knowledge of the molecular and cell biology of
the breast and breast tumours are giving new insights into potential
mechanisms of endocrine sensitivity and resistance. The normal breast
is relatively resistant to the major stimulatory hormone oestradiol
possibly because of the separation between the non-dividing ER positive
cell and adjacent proliferating ER negative cell.1 An early event
in the malignant process is the ability of the ER positive cell
to divide and adapt to the prevailing serum oestradiol concentration.2
The Oxford overview and the other studies indicate that the ER (or
PR) is a prerequisite for endocrine responsiveness.
A major research focus has been on the mechanism of resistance
to the antioestrogens. However, with an increase in the importance
of aromatase inhibitors, it is important to consider resistance
to them when detected. When detected, endocrine responsive breast
cancers are growing in response to the prevailing serum concentration
of oestrogens particularly oestradiol (E2). Reducing (or increasing)
the concentration of E2 or blocking its interaction with ER inhibits
tumour cell growth. In the adjuvant situation some micrometastases
appear to be inhibited lifelong by this approach whereas in advanced
breast cancer objective responses or stabilisation of disease occurs
for a finite period. Mechanisms of resistance are studied in the
clinic and in breast tumour cell lines and animal models. Studies
have reported a large number of potential mechanisms of resistance
but we need to focus on the most promising. These show alteration
of cell signalling pathways to the ER as a major cause of resistance.
For example, by depriving MCF-7 cells of E2 in-vitro Santen and
his colleagues3 have shown that the cells adapt and grow in response
to very low concentrations of E2 but also the signal transduction
enzyme is phosphorylated and can stimulate growth in an ER dependent
and non-dependent ways. Treating these cells with physiological
concentrations of E2 causes apoptosis and may be analogous to responses
to high dose oestrogens in-vivo. Reversible tamoxifen resistance
to tamoxifen may be related to growth factor activation of intracellular
mediators (eg, AKT2 and MAPK)4,5 which can phosphorylate the ER
and cause growth stimulation in the presence of tamoxifen-occupied
ER. Another mechanism of resistance to both tamoxifen and fulvestrant
(ICI 182,780) is seen when MAPK inhibits (by phosphorylation) the
activity of p27, a protein which blocks cell cycle progression.
Recent studies indicate that antioestrogens are inactive when p27
is low or inactivated.6 Antioestrogens and high dose oestrogens
may actively stimulate tumour growth in patients. One potential
mechanism is binding of the AE/ER complex to other transcription
factors (fos and jun) which may then secondarily stimulate growth
via API sites in the promotor regions of growth regulatory genes.
The importance of the cell and molecular biology studies concerning
mechanisms of resistance are that we can see methods for abrogating
them, for example, by adding signal transduction inhibitors to endocrine
therapy.
- Clarke et al, Cancer Research 57: 4987, 1997
- Shoker et al, Am J Pathol 155:1811, 1999
- Song et al, JNCI 93: 1714, 2001
- Kurokawa et al, Cancer Research 60: 5887, 2000
- Mei et al, Cancer Research 61: 5985, 2001
- Donovan et al, J Biol Chem 276: 40888, 2001
- Paech et al, Science 227: 1508, 1997
The potential mechanisms of four clinical scenarios will be discussed:
1. Response to tamoxifen and a second response to an aromatase inhibitor
(AI) or fluvestrant (ICI 182,780: Faslodex). 2. Response to a single
endocrine therapy and no response to a second therapy. 3. Response
to an AI and then oestrogen and 4. Antioestrogen stimulated growth.
1. Failure after initial response to tamoxifen is associated with
increased intratumoural expression of EGFR, c-erbB2, TGF( and activated
(phosphorylated) MAP kinase. Tamoxifen occupied ER can be phosphorylated
serine 118 by the activated MAP kinate3 pathway and on serine 167
by activation of the p13 kinase/AKT pathway4 via cell surface growth
receptors. In-vitro studies show that tamoxifen is ineffective when
these pathways are activated but active when the pathways are specifically
inhibited. Both fulvestrant and AIs prevent ER dimerisation receptor
phosphorylation suggesting this may be the mechanism of a second
response. 2. For response to antioestrogens, it is necessary for
the cell cycle inhibitor p27 to bind to cyclin E1. In-vitro experiments
indicate that activated MAP kinase inhibit p27 activity and produces
complete endocrine resistance to both tamoxifen and to fulvestrant.5
3. AIs lower serum oestradiol. Recently it has been shown in MCF-7
cells in-vitro and in the nude mouse model that the cell response
to low E2 is to increase ER concentration and activity. Importantly
it was shown that relatively high concentrations of E2 caused Fas
mediated apopotosis and may be the mechanism of response to high
dose oestrogens.6 4. Withdrawal responses to tamoxifen and high
dose oestrogens have been reported suggesting both therapies may
stimulate cell growth. Several groups have shown increased fas/jun
activity at API sites in tamoxifen resistant tumours and in-vitro
antioestrogens stimulate growth through this mechanism in the presence
of ER( and ER(.7 Elucidation of the molecular biology of endocrine
resistance is exciting and highly important since it is then possible
to devise ways to abrogate resistance using other inhibitors.
Back | Top of Page

|
|



