|
| Should axillary dissection be done? Richard
Margolese, M.D., Herbert Black Professor of Surgical Oncology,
McGill University, Montreal, Canada |
 |
Since the introduction of adjuvant chemotherapy for breast cancer,
lymph node status has been a defining indicator for selection of
patients and selection of therapy. Less invasive biochemical and
cellular markers have been studied and proposed as alternatives
but in every multivariate analysis lymph node status continues to
be the most reliable indicator for the risk of future metastasis.
With the establishment of benefit of adjuvant chemotherapy for
node negative women it appears that the majority of patients with
breast cancer will benefit from chemotherapy. The patients who are
unlikely to benefit from chemotherapy are those with small tumors
with favorable histology discovered on mammography. The yield from
axillary dissection runs between 5 and 15% on such tumors. If all
such women were subjected to axillary node dissection and 10% had
positive nodes and 20% of these had their prognosis altered by chemotherapy
then 98% would have the procedure with no chance for benefit.
Sentinel node biopsy is a less invasive procedure with almost the
same accuracy but the long term consequences of SNB are still to
be determined by ongoing clinical trials. It is likely that refinement
of prognostic features in genetic array analysis will ultimately
provide the best indicators for who should receive chemotherapy
and who is likely to benefit from it.
| Axillary Sentinel Lymph Node Biopsy: Hints
for the Neophyte Gordon F. Schwartz, M.D., MBA, Professor of
Surgery, Jefferson Medical College, Philadelphia, PA |
 |
Few procedures have been so rapidly adopted into clinical practice
as axillary sentinel lymph node biopsy (SLNB) in patients with breast
cancer. Despite criticisms that SLNB has not been validated by clinical
trials, its advocates maintain that, as a diagnostic procedure,
it does not require the same randomized trials as new treatments
would demand. Which of these positions is valid is moot, since SLNB
has been adopted throughout the world, and current major concerns
relate to perfecting its use.
Without preempting the subsequent discussion on technique, what
are some guidelines for those surgeons who have not yet embraced
SLNB, or for those who have had some difficulty in identifying “the”
sentinel node(s)? The sentinel node is defined as the first node
to which lymph drainage and metastasis from breast cancer occurs.
Usually an axillary node in level I, it may, however, be behind
the pectoralis minor muscle (level II), or even subclavicular (level
III). It may be an intramammary node, an internal mammary node,
or even a supraclavicular node, but these latter locations are rare.
With suitable training, SLNB is a replacement for axillary dissection
as a staging and diagnostic procedure in patients with T-1 and T-2
(usually 3 cm or less), N-0, M-0 cancers. Identification of sentinel
nodes in appropriately selected patients is >95%. When the sentinel
node, as identified by radiocolloid or blue dye, is contiguous to
node(s) that are clinically suspicious because of character or size,
they should be removed with the sentinel node(s).
Although the pioneers in SLNB were self-taught, performing SLNB
along with traditional axillary dissection to validate their own
techniques, current clinical trials (e.g. NSABP, ACOGOG) mandate
a training period with documentation of results before embarking
on SLNB without concomitant axillary dissection. Hospitals have
not addressed credentialing issues in SLNB as they have for other
procedures, e.g., laparoscopic surgery or stereotactic breast biopsy.
Until surgeons have documented a detection rate of >90% and
a false negative rate of <5%, they should perform concomitant
levels I/II axillary dissection. When radiocolloid is used, institutional
nuclear medicine teams must be involved, and in all cases, the surgical
pathologist must adopt special techniques to handle these specimens.
The precise number of SLNB procedures with concomitant axillary
dissection required to validate technique remains contentious; recommendations
range from 10 to 100 procedures. Often neglected as these numbers
are discussed is the important point that it is the number of patients
with positive nodes that will determine the false negative rate.
Since only ~30% of women with N-0 axillae will actually have node
metastasis, it may take a very large number of patients for any
single surgeon to validate his false-negative rate.
Whether radiocolloid, blue dye, or both are used to find sentinel
nodes is an individual decision made by the surgical team. Although
surgeons new to SLNB are advised to learn and use both radiocolloid
and dye to achieve the best results, as experience is gained, one
or the other may be used exclusively. (When a single technique is
used, it is usually blue dye alone.) Peritumoral injection is the
most commonly used site, but a combination of intradermal and peritumoral
sites is proving most effective. Retroareolar injection is still
considered experimental.
There are specific contraindications to SLNB. These include patients
with clinically positive axillae (N-1), since the anatomy of the
lymphatics may no longer be intact and lead the dye astray. Thus
far, patients who undergo induction chemotherapy for large tumors,
even with N-0 axillae, are not considered candidates for SLNB outside
a clinical trial. Allergic reactions to both dye and radiocolloid
have been reported; allergy to cosmetics containing blue dyes is,
therefore, a relative contraindication. Until more data are available,
SLNB should not be performed in pregnant women. Prior axillary procedures,
such as axillary node biopsy, augmentation mammoplasty using an
axillary incision, are relative contraindications. The performance
characteristics of SLNB in women who have undergone prior reduction
mammoplasty are unknown. A prior biopsy procedure, irrespective
of its character, i.e., excisional, incisional, or core, that proved
the diagnosis of cancer, is not a contraindication to SLNB and does
not affect its success. This question had previously arisen as the
practice of SLNB evolved. The role of SLNB in multicentric cancer
has not been established. For two discontiguous tumors, two separate
injections, one at each tumor site, have been used effectively.
Whether frozen section of lymph nodes is performed is an institutional
decision. Most experienced surgeons use frozen sections to determine
the presence of metastasis, so that if a more complete axillary
dissection is required, it can be performed at the same time as
the sentinel node biopsy. Each institution should have its own established
pathology protocol. The College of American Pathologists has already
promulgated one such protocol. Immunochemical (cytokeratin) staining
of axillary nodes is often performed although this information should
not be used to influence therapeutic decisions. Although “sub-microscopic”
metastasis, i.e. CK-positivity only, has been proved, no data are
available to suggest that the clinical outcome of patients with
these sub-microscopic metastases is different from those with CK-negative
nodes.
The role of SLNB in DCIS is another contentious point. DCIS alone,
i.e., without evidence of microinvasion, is not an indication for
SLNB. However, patients with DCIS and microinvasion (T-1-mic) should
be considered for SLNB.
Patients undergoing mastectomy may be candidates for SLNB, using
the same criteria as for patients undergoing breast conservation.
The argument for frozen section is more valid in these patients,
since completion axillary dissection following mastectomy is more
technically difficult than it would be if it were done at the same
time as mastectomy.
| Technique of Sentinel Node Biopsy: Methods
to Reduce False Negative Rates Patrick I. Borgen, M.D., Chief,
Breast Service, Department of Surgery, Memorial Sloan-Kettering
Cancer Center, New York City, NY |
 |
Sentinel lymph node biopsy has established itself as an acceptable
standard of care, in the hands of the experienced surgeon, for staging
the axilla in breast cancer. The basic hypothesis is that there
is a first (sentinel) lymph node that represents the overwhelmingly
most likely site of metastasis to the regional node-bearing area.
The hypothesis contends that this node should be an accurate representative
for the status of the balance of the nodes in the basin. That is,
if this node is negative, it should not be necessary to proceed
with removal of additional nodes. Sentinel lymph node studies to
date, which include over 50 published institutional experiences
using a variety of techniques and in a variety of disease situations,
have strikingly similar results suggesting that the hypothesis has,
in fact, been verified. In the United States, sentinel lymph node
biopsy is rapidly gaining popularity and acceptance. Studies of
sentinel lymph node biopsy focus on two outcomes. The success of
a sentinel lymph node biopsy is defined as finding a hot or blue
(or both) lymph node. In cases in which isotope is used, success
is further defined by achieving a significant reduction in background
isotope counts after removal of the sentinel lymph node. The second
parameter, and in many ways the most important parameter, is the
accuracy. The accuracy in sentinel lymph node biopsy is defined
as the false-negative rate. It is very important to define the false-negative
rate calculation as the cases with (missed) positive nodes divided
by the number of cases of proven node negatives (in patients who
have had an axillary clearance). The largest series published to
date report false-negative rates in the 3-8% range. This range of
false-negativity has been quite disturbing many. It is important,
therefore, to remember that sentinel lymph node biopsy is not being
compared against perfection. A full axillary dissection has a known
12-20% false-negative rate when lymph nodes that were signed out
as negative are reevaluated more closely. Therefore, the false-negative
rate with sentinel lymph node biopsy, in experienced hands, is lower
than the false-negative rate associated with axillary node dissection.
Strategies to reduce the false-negative rate can be divided into
several categories. These are patient selection criteria, tracer
blue dye selection/site of injection, intraoperative strategies
and postoperative decision-making. Each of these is handled separately
below.
Patient Selection Criteria
As the evidence mounts validating the sentinel hypothesis, a broader
and broader category of patients appears to be appropriate candidates
for sentinel lymph node biopsy. However, several groups of patients
remain questionable candidates for this procedure. Patients with
multiple invasive primary cancers in at least two separate quadrants
may have a higher false-negative rate and current thinking is that
they should be excluded from sentinel lymph node biopsy alone. Ongoing
research may reveal that the entire breast has a sentinel node,
in which case these cases would not be excluded. Secondly, patients
with locally advanced cancer may have bulky axillary disease. It
is likely that in some cases, a lymph node that is replaced by breast
cancer will have reduced or non-existent lymphatic flow, thus increasing
the likelihood of false-negativity. Similarly, patients who have
had induction chemotherapy prior to surgery may have had the sentinel
lymph node partially or totally sterilized by the chemotherapy,
while other nodes in the axilla may contain viable metastatic disease.
Along the same lines, patients with clinically suspicious ipsilateral
axillary lymph nodes are also questionable candidates for a sentinel
lymph node biopsy and at the very least, these nodes should be sampled
if they are not the sentinel lymph node. Patients with tumors that
are quite large and quite high in the axillary of Spence represent
a special technical challenge as the distance to be mapped can be
quite small. Tracer injection site overlay may mask the true sentinel
node; blue dye may permeate the lower lymphatics and nodes.
Choice of blue dye/tracer and site of injection
Sentinel lymph node biopsy has been shown to be accurate using
a variety of techniques and a variety of dyes and tracers. Blue
dye and radiotracer used in Europe is often different from the blue
dye and tracer used in the United States. Colloidal albumen is a
radiotracer that has enjoyed great success in Europe but is not
approved in the U.S. A number of individuals and centers are quite
proficient with blue dye or tracer alone and have published excellent
results with their technique. There is mounting evidence that the
combination of blue dye and tracer results in the highest success
rate and the lowest false-negative rate (McMasters, et al). Moreover,
there is mounting evidence that intradermal injection site of tracer
is associated with a significantly better sentinel lymph node identification
rate than either subdermal or peritumoral injection (Linehan, McMasters).
It is reasonable and likely that the combination of blue dye and
tracer results in the largest number of nodes being identified.
Our group has previously reported that 25% of disease in sentinel
lymph nodes is found in the second third or fourth sentinel lymph
node removed. The addition of radiotracer may play its biggest role
in the identification of multiple sentinel lymph nodes. This procedure
can certainly be done with blue dye, but in our hands is facilitated
using the hand held gamma probe and the radiolabeled tracer. It
is also true that substantial reduction in background counts, post-removal
of all sentinel lymph nodes, is quite reassuring in terms of the
search for additional lymph nodes. In our hands, this has had a
beneficial effect on our published false-negative rates.
Intraoperative Decision Making/Technique
It is more valuable to identify a failed sentinel lymph node procedure
than it is to confirm a successful one. When using radiotracer,
a failed procedure is defined as one in which there is no identifiable
radioactivity in the axilla or when there is diffuse radioactivity
covering much of level I and/or level II. Fragmentation of the radiolabeled
colloid can lead to a smaller particle size, which can permeate
the axilla and make identification of the appropriate node problematic.
Either of these scenarios should lead to consideration of a completion
axillary dissection. In cases where tracer and blue dye are both
used, we attempt to use blue dye to salvage the sentinel node biopsy
procedure. If blue lymph nodes are identified, we will accept those
as sentinel lymph nodes. The value of visually inspecting and digitally
palpating the other nodes in the axilla cannot be overstated. It
is very likely that lymph nodes that are replaced with breast cancer
cells will have an attenuated lymphatic flow. This means that the
nodes we are most interested in finding may not contain blue dye
or tracer but may be palpable. We have documented a substantial
reduction in our institutional false-negative rate with this simple
maneuver. Any node that is felt to be unusually large or have an
unusually large consistency is removed whether it is the sentinel
lymph node or not. Palpating extra anatomic sites such as Rotter’s
space in level II can also reduce false-negatives as sentinel lymph
nodes in these locations have been reported. Patients with very
large primary tumors, particularly high-grade cancers with extensive
lymphovascular invasion, are also candidates for additional node
removal (in addition to the sentinel lymph node). These are patients
with a high likelihood of axillary disease and it is not unreasonable
to sample additional lymph nodes in hopes of lowering the false-negative
rate.
The most important technical lesson we have learned is to carefully
proceed layer by layer in the axilla carefully controlling small
perforator vessels. Blood staining the operating field makes identification
of fine blue lymphatics very difficult and can even make the determination
of whether a node is blue or not quite difficult. This should be
a careful anatomic dissection, not an exercise in blunt dissection
and ‘rooting’. Paying attention to hemostasis early in
the case also significantly shortens operative time. Without question,
the false negative rate can be impacted by following basic surgical
principles.
Postoperative Decision Making
Unanticipated findings on the final pathology either on the breast
tumor specimen or the axillary nodes may warrant further surgery.
If we define a false-negative as leaving any positive lymph nodes
in the axilla, then it would follow that any positive sentinel lymph
node should lead to further axillary node removal. Twenty to thirty
percent of patients with a positive sentinel lymph node are found
to have additional positive non-sentinel lymph nodes in the nodal
basin. At least six papers published to date have evaluated potential
prognostic indicators for predicting non-sentinel lymph node positivity.
All studies to date have been unable to reliably do so and all have
concluded that a completion axillary dissection should remain the
standard of care. A number of important ongoing trials are addressing
this issue more closely and in more detail. Considerable debate
exists about whether a lymph node with a metastasis seen on IHC
only should lead to completion axillary burgery. This remains an
unanswered question and again, ongoing trials have been designed
to address the issue. Data will be presented concerning our own
institutional experience with and approach to micrometastases.
- Chu KU, Turner RR, Hansen NM, et.al. Do all patients with sentinel
node metastasis from breast carcinoma need complete axillary node
dissection? Ann Surg 1999; 229:536-541.
- Weiser MR, Montgomery LL, Tan LK, et.al. Lymphovascular invasion
enhances the prediction of non sentinel node metastases in breast
cancer patients with positive sentinel nodes. Ann Surg Oncol 2001;
8:145-149.
- Reynolds C, Mick R, Donohue JH, et.al. Sentinel lymph node
biopsy with metastasis: can axillary dissection be avoided in
some patients with breast cancer? J Clin Oncol 1999; 17:1720-1726.
- Chu KU, Turner RR, Hansen NM, et.al. Sentinel node metastasis
in patients with breast carcinoma accurately predicts immunohistochemi-cally
detectable nonsentinel node metastasis. Ann Surg Oncol 1999; 6:756-761.
- Turner RR, Chu KU, Qi K, et.al. Pathologic features associated
with nonsentinel lymph node metastases in patients with metastatic
breast carcinoma in a sentinel lymph node. Cancer 2000; 89:574-581.
- Wong SL, Edwards MJ, Chao C, et.al. Predicting the status of
the nonsentinel axillary nodes: a multicenter study. Arch Surg
2001; 136:563-568.
- Linehan D, Hill A, Akhurst T, Yeung H, et a, Intradermal radiocolloid
and intraparenchymal blue dye injection optimize sentinel node
identification in breast cancer patients. Ann Surg Onc 1996(5):450-454.
- Cody HS, Hill AD, Tran K, Brennan M, Borgen PI, Credentialing
for breast lymphatic mapping - how many cases is enough? Ann of
Surg 1999; 229(5):723-728
- Derosis A, Fey J, Yeung H, et al, A trend analysis of the relative
value of blue dye and isotope localization in 2000 consecutive
cases of sentinel node biopsy for breast cancer. J Am Coll surg
2001; 193:473-478
| The UK sentinel node trial (ALMANAC) Robert
E. Mansel, MS, Professor of Surgery, University of Wales College
of Medicine , Cardiff, UK |
 |
Sentinel node biopsy has become a popular technique in many breast
units throughout the world, despite the fact that as yet no data
has become available from randomised trials. Several large trials
are underway in the US, notably the B-32 from the NSABP and American
College of Surgeons trials Z10 and Z11. Within Europe the largest
current randomised trial is the ALMANAC study, which had randomised
over 500 patients by December 2001 in 14 UK centres. The trial is
similar in structure to the B-32 with randomisation between conventional
axillary surgery and sentinel node biopsy alone with further treatment
been given only to the positive sentinel node containing axilla
by either further surgery or radiation therapy.
The UK trial is unique in that all surgeons were required to pass
a predetermined level of competence in performing sentinel node
biopsy, after a period of in-hospital training by the principal
investigator. Only those surgeons who achieved a greater than 90
percent localisation rate and a false negative rate of equal or
less than 5% over 40 audit cases were permitted to enter patients
into the randomised phase. In the preliminary audit phase of over
800 patients, in which all patients underwent formal axillary staging
after sentinel node biopsy, it was notable that a small learning
curve was recorded.
Primary endpoints of the trial are quality of life measures, health
economics and arm morbidity. Local occurrence will also be a secondary
endpoint. A new axillary subscale of the Fact B-4 quality of life
questionnaire has been developed and validated for this trial. Early
analysis of the audit data is is is shows the subscale is sensitive
in discriminating between axillary clearance and sampling procedures.
We have found that an increased BMI and age are risk factors for
failed localisation, but multifocal tumours or previous surgery
had no effect. The randomised trial continues in progress.
| Sentinel Node Biopsy - Early Results of
a Randomized Trial Umberto Veronesi, M.D., Scientific Director,
European Institute of Oncology, Milan, Italy |
 |
In most patients presenting today with small breast cancer the
axillary lymph nodes are free of cancer cells at histological examination,
so that routine axillary dissection appears an overtreatment. One
objective of breast cancer research is to provide the surgeon with
preoperative information on axillary node status, so that axillary
dissection can be avoided if the nodes are negative.
The sentinel node biopsy (SNB) methodology has been developed for
this purpose, and numerous studies have shown that the status of
the biopsied node is an acceptably accurate predictor of the status
of the other axillary nodes (1,2,3). Although these findings are
encouraging, the method still requires validation in terms of efficacy
and safety.
We therefore decided to carry out a randomized study comparing
two series of patients, one treated with routine axillary dissection
and the other with the SNB policy. This policy is that if the sentinel
node is negative at intraoperative histological examination no further
surgery to the axilla is performed.
The study was approved by the Ethics Committee of the European
Institute of Oncology. It was a single center, randomized clinical
trial with two arms. Patients with primary breast carcinoma less
than 2 cm were randomly allocated, after breast conserving surgery,
to either sentinel node biopsy and total axillary dissection (AD
arm) or sentinel node biopsy followed by axillary dissection only
if the sentinel node was metastatic ( SN arm). The SNB procedure
was identical in both arms.
As SNB is a diagnostic and staging procedure, the primary end-point
(a) of the study was to determine its staging power or percentage
of cases with axillary involvement in relation to the percentage
found by routine axillary dissection. Additional end-points were
(b) patients’ quality of life in the two arms, (c) the number
of overt axillary lymph node metastases appearing in SN arm patients
with a negative sentinel node and, (d) long-term disease-free and
overall survival in both arms.
Patients over 40 and less than 75 years of age, with invasive breast
carcinoma less than 2 cm in maximum diameter and no previous history
of malignancy, were eligible. Breast cancer was diagnosed from clinical
examination, mammography or ultrasonography and, in most cases,
a positive fine needle biopsy. Patients with multicentric cancer
or who had undergone previous excisional biopsy were not eligible.
Patients were randomized in the operating theater after the size
and histology of primary carcinoma had been ascertained.
We considered a series of 649 consecutive patients initially. Of
this initial series 532 were randomized. Of the 532 randomized patient
16 were not evaluable.
Most patients (410, 79%) were injected with radio-tracer the day
before surgery, the remainder (106 patients, 21%) were injected
the same day. Five to ten MBq of 99mTc-labeled colloidal human albumin
particles (size range 50-200 nm) in 0.2 mL of saline were injected
close to the tumor (4). Injection was subdermal if the tumor was
superficial and peritumoral if it was deep. Anterior and anterior-oblique
lymphoscintigraphic projections of the breast and axilla were subsequently
obtained to reveal the dynamics of lymphatic flow (in particular
whether more than one lymph node took up tracer) and to determine
the exact position of the sentinel node. The skin projection of
the sentinel node(s) was drawn to serve as landmark during biopsy.
In eight (1.2%) of the 649 patients of the original series there
was no uptake of the radiotracer at scintigraphy and these were
not considered eligible for the trial.
Of the 516 evaluable patients, 292 had one sentinel node, 152 had
two sentinel nodes, 47 had three sentinel nodes and in 25 more than
three were identified. All axillary nodes taking-up radiotracer
were removed and classified as sentinel nodes and all were evaluated
histologically. The distribution of number of sentinel nodes found
did not differ in the two arms. A total of 429 sentinel nodes were
removed and examined from AD arm and 424 from SN arm. The total
of 6200 axillary lymph nodes were removed (including sentinel nodes)
from the 257 patients of AD arm (average 24 per axilla) and a total
of 2249 were removed from the 93 patients of SN arm who had positive
sentinel nodes (average 24 per axilla).
The first end-point of the study was to assess the ability of the
sentinel node policy to screen cases with a positive axilla, in
comparison with the detection rate in patients who received total
axillary dissection. Among the 257 patients of AD arm, a sentinel
node was positive in 83 (32.3%) cases and negative in 174 (67.7%);
among the 259 patients of SN arm, a sentinel node was positive in
93 (35.9%) and negative in 166 (64.1%).
Among the 257 patients of AD arm all of whom received total axillary
dissection, there were eight cases with metastatic axillary nodes
but a negative sentinel node; in two of these cases there were micrometastases
in one axillary node only. The overall accuracy of the SN technique
in AD arm was therefore 96.9%, the positive predictive value was
100%, the negative predictive value was 95.4%, the sensitivity 91.2%,
and the specificity 100%.
Out of 176 patients with metastatic sentinel nodes in 60 micrometastases
only were found, defined as one focus of metastatic cells less than
2 mm in diameter. In 41 of these 60 cases the focus was smaller
than 1 mm. In AD arm, 29 patients had a micrometastatic sentinel
node, in 24 of which all the other axillary nodes were negative,
while in five, one other axillary node was positive. In SN arm,
31 patients had a micrometastatic sentinel node, in 26 of which
all other axillary nodes were negative, while in 5 there was one
positive node. The median follow up for each patient is 26 months
and the total person years on study is 552 in the AD arm and 566
in the SN Arm. There are 9 events associated with Breast Cancer
to date, 8 in the AD arm and 1 in the SN arm. In the AD arm there
are 2 controlateral breast cancers, 2 bone metastases, 2 lung metastases
and 2 patients with multiple metastases. One patient in the SN arm
had a bone metastases. Five patients, all in the AD arm, have died.
Two due to metastases from breast cancer, one for carcinoma of the
pancreas, one for endometrial carcinoma and one for intercurrent
disease.
References
- Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping
and sentinel lymphadenectomy for breast cancer. Ann Surg 1994;
220:391-401.
- Krag D, Weaver D, Ashikana T, et al. The sentinel node in breast
cancer - a multicenter validation study. New Engl J Med 1998;
339(14), 941-946.
- Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node
biopsy to avoid axillary dissection in breast cancer with clinically
negative lymph-nodes. Lancet 1997; 349:1864-1867.
- De Cicco C, Paganelli G, Cremonesi M, et al. Lymphoscintigraphy
and radioguided biopsy of the sentinel axillary node in breast
cancer. Eur J Nucl Med 1998; 39 (12): 2080-2084.
| Identification of Metastatic Axillary and
Internal Mammary Nodes with MRI and Contrast Media Steven E.
Harms, MD, FACR, Professor of Radiology, University of Arkansas
for Medical Sciences, Little Rock, AR, and Medical Director,
Aurora Imaging Technology, North Andover, MA |
 |
Introduction:
The presence of axillary node metastasis is the most reliable predictor
of of outcome in women with breast cancer. Therefore, axillary node
dissections have become a routine part of breast cancer staging.
With early cancer detection, the occurrence of positive lymph nodes
is declining. Recent studies indicate that only about 10% of patients
with invasive cancers of 1 cm or smaller in size will have metastatic
nodes.1,2,3 This represents an improvement from the late 1960’s
when about one third of small cancers had nodal metastases. Although
node dissections have prognostic benefit, there is no therapeutic
benefit for most patients. The relatively high morbidity of axillary
dissection relative to its benefit have prompted efforts to avoid
this surgery in women with other signs favoring a good prognosis.2,3
Sentinel node surgery is becoming a popular alternative to traditional
axillary node dissections. A non-invasive method of accurately predicting
nodal metastasis could further reduce costs and morbidity while
potentially improving nodal sampling beyond the sentinel nodes.
Ferumoxtran-10 (F-10) is a biodegradable, ultrasmall, superparamagnetic
iron oxide covered with a low molecular weight dextran. When given
intravenously, it shows active uptake by the reticuloendothelial
system (liver, spleen, lymph nodes, and bone marrow). The particle
size is such that there is preferential uptake by lymph nodes. After
twenty-four hours, normal nodes contain a high concentration of
F-10. This results in marked T2*-shortening (magnetic susceptibility
effects) and a significant loss of signal within the node on a T2*
weighted MRI image. Tumor-associated macrophages within and around
primary and metastatic foci have less avid uptake and demonstrate
low concentration T1 shortening effects. Metastatic foci within
nodes increased intensity on T1-weighted images.4-7
Purpose:
The objective of this study is to determine the potential for ferumoxtran-10-enhanced
MRI to localize axillary metastases in women with invasive breast
carcinoma. Results of ferumoxtran-10 MRI will be compared with more
conventional gadolinium-enhanced MRI for differentiating metastatic
from nonmetastatic nodes, using histologic confirmation.
Methods:
Patient selection:
All women in the study were above 18 years of age and were recently
diagnosed with a biopsy proven breast carcinoma less than or equal
to 3 cm in size. All patients underwent breast conservation treatment
and subsequent axillary lymph node sampling by conventional node
dissection or by sentinel node biopsy. At this time a total of 27
patients from 7 institutions have been studied.
Imaging:
All studies in the multi-institutional trial employed routine 2D
T1-weighted and T2 weighted spin-echo and T2* gradient echo sequences.
In addition to the routine protocol, images at our institution were
obtained on a 1.5 Tesla MRI imager (General Electric, Milwaukee)
using the 3D RODEO (Rotating Delivery of Excitation Of-resonance)
pulse sequence. The 3D scans provided improved spatial and contrast
resolution to demonstrate the potential benefit of future image
enhancements on overall contrast agent performance.
Drug administration:
Ferumoxtran-10 (2.6 mg Fe/kg) was administered intravenously after
diluting the agent with 50 ml of normal saline (NaCl, 0.9%) at a
rate of 4 ml/min. No adverse affects were reported.
Procedure:
Pre- and post-contrast gadolinium enhanced images were obtained
in 5 cases. In all cases pre-, immediate, and 24-36 hour delayed,
post-ferumoxtran-10 MRI images were obtained and analyzed. All lymph
nodes were categorized according to size, anatomic location, and
diagnosis (normal, metastatic, possibly metastatic) An axillary
lymph node dissection bwas performed within 48 hours of administration
of F-10.
Results:
Surgical specimens, obtained shortly after ferumoxtran-10 administration
and examined with specific iron stains, demonstrated high concentrations
of iron within normal nodes consistent with uptake of the agent
. This high concentration of iron correlates with the intense T2*
shortening (hypointensity) due to magnetic susceptibility effects
on T2* weighted MRI scans. Metastatic and primary tumors showed
low concentrations of iron within tumor associated macrophages(Figure
1). Low concentrations of iron result in hyperintensity on T1 weighted
MRI scans.
The summary data from the multicenter trial of overread images
combining the results of 2 blinded readers are available in table
1. Using size criteria alone, the widely used standard in CT and
MRI currently, the specificity is high (96%) but the sensitivity
is low (27%). The post-ferumoxtran-10 images provided nearly equivalent
specificity (91%) but significantly improved sensitivity (68%) and
accuracy (78%). This improvement is attributed to the ability to
detect normal sized metastatic nodes. These results demonstrate
the potential for ferumox-tran- 10 lymph node specific contrast
to depict nodes that would otherwise be missed on conventional MRI
scans.
Table 1
| Evaluation |
#of Nodes |
Sensitivity % |
Specificity % |
Accuracy % |
PPV % |
NPV % |
| Size |
93
|
27
|
96
|
74
|
77
|
74
|
| Pre-dose |
93
|
78
|
49
|
58
|
48
|
90
|
| Post-dose |
97
|
68
|
91
|
84
|
79
|
85
|
| Paired |
92
|
78
|
77
|
78
|
63
|
89
|
Despite the ability of ferumoxtran-10 to deliver sufficient contrast
between normal nodal tissue and metastatic disease, a major limitation
of current MRI is the ability to generate sufficient resolution
to depict small lymph node metastases. The multicenter trial employed
standard MRI pulse sequences with 5 mm thick sections . At UAMS,
we also used the RODEO pulse sequence, which allowed the generation
of 500 micron section thicknesses. In addition, the combined T1
and T2* weighting of RODEO further enhances the contrast mechanisms
of ferumoxtran-10 (Figure 2).
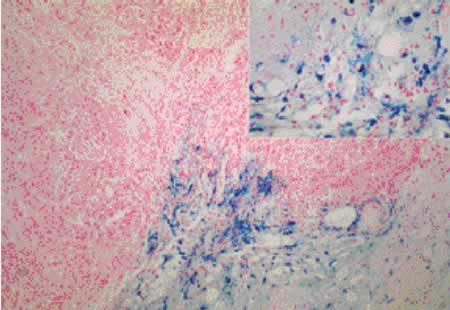
Figure 1: Iron stain within tumor-associated macrophages
The Prussian blue stain (high power insert) demonstrates blue staining
iron within macrophages surrounding and within an infiltrating breast
carcinoma. This effect correlated with the hyperintense signal on
T1 weighted MRI images from low concentration uptake of ferumoxtran-10.
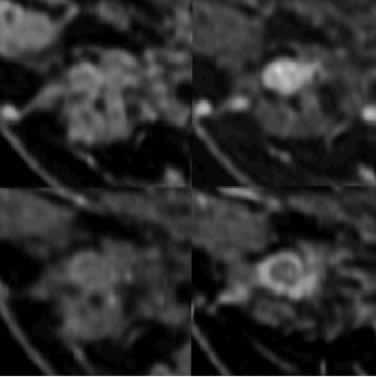
Figure 3: Axial reformatted pre- (A) and post-gadolinium (B) RODEO
images of the axilla, of the same patient as Figure 3, demonstrate
multiple iso-intense to hyper-intense lymph nodes of various sizes
but of similar morphology. Note the improvement in resolution compared
to standard imaging techniques.
Immediately (C) and 24 hours (D) after the administration of F-10,
there is progressive iron uptake within normal axillary lymph nodes
resulting in low signal intensity (small yellow arrows). These smaller
nodes are more clearly identified on the RODEO sequence. A malignant
node (large red arrow) demonstrates no uptake and remains high in
signal intensity. The largest node (blue arrow) was only partially
involved with metastatic disease and has components of high and
low signal.
Conclusion:
This study demonstrates the potential for ferumoxtran-10 enhanced
MRI in the evaluation of axillary nodal metastases from breast cancer
in normal sized nodes. When the contrast and spatial resolution
is optimized with RODEO MRI, significantly improved nodal sampling
can be achieved with section thicknesses approaching 500 microns.
This capability could rival the sampling ability of conventional
node dissections in a non-invasive examination.
Non-invasive node evaluations will be most helpful in patients
with small infiltrating carcinomas and DCIS where the probability
of node involvement is small. A non-invasive test that could exclude
nodal metastases would lower morbidity and reduce health care costs.
If positive, newly developed MRI stereotaxic methods can be used
to selectively sample nodes without a node dissection.
The use of ferumoxtran-10 enhanced RODEO MRI is expected to produce
significant improvements in the management of breast cancer in the
future.
Reference:
- Reger V, Beito G, Jolly PC. Factors affecting the incidence
of lymph node metastases in small cancers of the breast. Am J
Surg 1989; 157:501-502.
- Cady B, Stone MD, Wayne J. New therapeutic possibilities in
primary invasive breast cancer. Ann Surg 1993; 338-349.
- Silverstein MJ, Gierson ED, Waisman JR, et al. Axillary lymph
node dissection for T1a breast carcinoma: Is it needed? Cancer
1994; 73:664-667.
- Vassallo P, Matei C, Heston W, et al. AMI-227—enhanced
MR Lymphography: Usefulness for Differentiating Reactive from
Tumor-bearing Lymph Nodes. Radiology 1994; 193: 501-506.
- Anzai Y, McLachlan S, Morris M, et al. Dextran-coated Superparamagnetic
Iron Oxide: The First Human Use of a New MR Contrast Agent for
Assessing Lymph Nodes in the Head and Neck. Amer. J. Neuroradiology
1994; 15: 87-94.
- McLachlan S, Morris M, Lucas M, et al. Phase I Clinical Evaluation
of a New Iron Oxide MR Contrast Agent. Magnetic Resonance Imaging
1994; 4: 301-307.
- Anzai Y, Blackwell K, Hirschowitz S, et.al. initial clinical
experience with dextran-coated superparamagnetic iron oxide for
detection of lymph node metastases in patients with head and neck
cancer. radiology 1994; 192: 709-715.
| Journal of Clinical Oncology Special Issue
Review Daniel F. Hayes, M.D., Director, Breast Oncology Program,
University of Michigan Comprehensive Cancer Center, MI |
 |
I was asked by George Canellos to select the “Classic”
papers regarding breast cancer published in the Journal of Clinical
Oncology from 1990-2000 1. This was a daunting task for several
reasons. 1) Over 500 breast-related articles were published in the
journal during that time. 2) The definition of classic is very much
operator dependent. I chose the following definitions: a) publication
of the paper changed treatment (as far as I can tell); b) the concept
presented a fundamental observation regarding the natural history
of the disease; and/or c) the report represented the first observation
(to my knowledge) of what would turn out to be an important area
of research.
I divided the issue into the obvious categories (not unlike this
symposium): Adjuvant Therapy, Bisphosphonates, Metastatic Chemotherapy,
Metastatic Hormonal Therapy, Metastatic anti-HER2 therapy, Natural
History, Primary Therapy, Prognostic and Predictive Factors, and
Quality of Life. I am particularly pleased that at least three of
these (bisphosphonates, anti-HER2 therapy, and QOL) would not have
been considered in 1990. I excluded the reports that represented
updates of previously published studies, Reviews, and ASCO Guidelinies
publications (to my chagrin, but space constraints dictated this
decision).
I included papers that others did not feel were classic, and I
excluded some that are (to quote Yogi Berra, “Can’t win
for losin’ “). Mortality from breast cancer is dropping
in the Western World, and at the same time survivors are living
better lives 2. This special issue of the Journal reflects some
of the reasons for these advances, and we all owe thanks to the
many investigators and patients who have worked so hard to produce
these results.
- Hayes DF: Classic Papers and Current Comments: Highlights of
Breast Cancer Research, in Canellos GP (ed): Classic Papers and
Current Comments from the Journal of Clinical Oncology. Baltimore,
Lippincott Williams & Williams, 2001
- Peto R, Boreham J, Clarke M, et al: UK and USA breast cancer
deaths down 25% in year 2000 at ages 20-69 years. Lancet 355:1822.,
2000
| Current and Proposed Trials of Local Therapy
Dr. Tim Whelan, B.M., B.Ch., M.Sc., Associate Professor, Dept.
of Medicine and Director, Supportive Cancer Care Research Unit,
McMaster University, Ontario, Canada |
 |
Following the seminal trials in the 1980’s demonstrating that
breast conserving surgery is equivalent to mastectomy for women
with early breast cancer, there have been a number of trials evaluating
the role of breast irradiation following lumpectomy. These studies
have uniformly demonstrated that radiation therapy reduces the risk
of local recurrence. Recently there have been a number of trials
evaluating the need for radiation in women at low risk of local
recurrence following lumpectomy. NSABP B21 studied the need for
radiation therapy in women with node negative breast cancer with
primary tumours less than 1 cm treated with Tamoxifen. The study
demonstrated that despite the use of Tamoxifen radiation resulted
in a significant decrease in local failure. Two other trials have
evaluated the role of radiation therapy in older women treated with
Tamoxifen. The CALGB trial studied women 70 years or older with
clinical Stage I disease and the Canadian trial studied women 50
years of age and older. The results of these studies are conflicting
but follow up is still short.
Other recent trials have evaluated how best to deliver radiation
therapy following lumpectomy including, accelerated treatment with
increased fractionation, and the role of additional boost treatment
in women with clear resection margins following lumpectomy.
Another area of recent interest has been the role of locoregional
radiation therapy. A number of randomised trials have demonstrated
that locoregional radiation following mastectomy in patients treated
with adjuvant systemic therapy not only reduces the risk of local
recurrence, but provides additional survival benefits in selected
patients. These trials were performed largely in women treated with
limited axillary dissection, who received non-anthracycline based
chemotherapy or relatively short duration hormonal therapy. Recently,
two randomized trials have been opened which should clarify the
role of locoregional radiation in the modern management of breast
cancer. The SWOG S9927 trial will evaluate locoregional radiation
post-mastectomy in patients with 1 to 3 positive axillary nodes
following adjuvant chemotherapy. The NCI(C) MA20 will evaluate regional
radiation in addition to breast irradiation in patients with high
risk node negative and node positive breast cancer.
In addition to these large phase III trials there are a number
of important Phase I & II trials evaluating different ways of
delivering adjuvant radiation therapy including brachytherapy, intraoperative
radiation and the use of intensity modulated radiation therapy.
Successful approaches will likely be taken into phase III testing
in the next few years.
| Current and proposed trials adjuvant therapy
for breast cancer Debu Tripathy, M.D., UCSF Carol Franc Buck
Breast Care Center, University of California, San Francisco,
CA |
 |
Stepwise improvements in the care and outcomes of patients with
breast cancer require a well thought out and deliberate set of clinical
trials. These trials must be based on biological underpinnings as
well as the experience from prior trials. Prioritization of trials
depend on the degree of enthusiasm for the new drug being tested,
such as the preliminary response rates seen in Phase II trials.
However, issues such as feasibility (number of patients needed and
safety of the drug/ease of administration) must be considered for
the trial to finish quickly and for the results to be valid and
useful. In the area of early stage breast cancer, several key issues
are currently under investigation:
* Addition of taxanes
* Optimal duration of chemotherapy (number of cycles)
* Sequential versus alternating/combination chemotherapy
* Dose-dense chemotherapy
* Neoadjuvant chemotherapy (types of agents and sequence)
* High dose chemotherapy with stem cell rescue for high risk disease
* Role of aromatase inhibitors in early stage breast cancer
* Role of oophorectomy in premenopausal women in addition to chemotherapy
* Postmastectomy radiation * Adjuvant bisphosphonates
* Choice of chemotherapy based on HER2/neu status and other markers
* Adjuvant anti-HER2/neu antibody (Herceptin)
* Vaccines * Prostaglandin inhibitors
* Diet/lifestyle modifications
Specific strategies and trials are discussed below
| The use of aromatase inhibitors (AI)
either in combination or in sequence (full 5 years or tamoxifen/AI
split over 5 years is being tested in many trials using letrozole,
anastrazole and exemestance. |
| Examples: |
TAM x 5 yr vs Tam x 5 yr ‡ letrozole x 5
yr |
| |
TAM x 5 yr vs TAM x 2-3 yr ‡ Exemestane 2-3 yr |
| Oophorectomy is also being studied
when added to chemotherapy and tamoxifen in premenopausal patients
with hormone receptor-positive breast cancer |
| Example: |
Chemo (or no chemo) then TAM x 5 yr +/- medical oophorectomy
x 2-3 yr. Some of these studies may be amended to also include
oophorectomy plus aromatase inhibitor arm |
| |
|
Substituting taxanes for alkylators is being studied in order to
eliminate some of the alkylator toxicities such as ovarian failure
and leukemia risk. Example: Intergroup study comparing doxorubicin
plus paclitaxel x 4 to standard cyclosphospamide plus doxorubicin.
The study is completed and results are pending
Adding a taxane remains of uncertain value since its addition to
doxorubicin plus cyclophosphamide resulted in improved disease-free
survival, yet a retrospective analysis suggested that this benefit
was confined to ER-negative cases. A confirmatory trial by the NSABP
will reported soon. An ongoing Canadian trials is also comparing
the addition of taxanes to an epirubicin-based regimen: CEF x 6
vs AC x 4 T x 4 vs EC x 6
T x 4 vs EC x 6 T x 4 (C=cyclophosphamide; E=epirubicin; F=fluorouracil; A=doxorubicin;
T=docetaxel;)
T x 4 (C=cyclophosphamide; E=epirubicin; F=fluorouracil; A=doxorubicin;
T=docetaxel;)
Prior trials have not clearly identified the optimal duration of
anthracycline or taxane-based therapies. Hence a trial is being
planned using a 2 x 2 designe that will compare AC x 4 vs AC x 6
vs weekly paclitaxel x 12 vs weekly paclitaxel x 18.
Using drugs in combination compared to using them in sequence.
Mathematical modeling has shown potential advantages to using drugs
in a dose-dense sequential fashion. Example: NSABP B-30 - AC x 4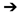 D x 4 vs AD x vs ADC (A=doxorubicin; D=docetaxel; C=cyclophosphamide)
D x 4 vs AD x vs ADC (A=doxorubicin; D=docetaxel; C=cyclophosphamide)
New combinations using capecitabine are also being planned. The
NSABP will compare AC 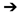 D vs AC
D vs AC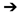 D + Capecitabine (A=doxoru-bicin; C=cyclophosphamide; D=docetaxel).
A trial for older women will compare CMF or AC to capecitabine
D + Capecitabine (A=doxoru-bicin; C=cyclophosphamide; D=docetaxel).
A trial for older women will compare CMF or AC to capecitabine
Bisphosphonates will also be tested following standard chemotherapy
and hormonal therapy. Observation will be compared to bisphosphonate
(clodronate in the NSABP study and zolendronate in the SWOG study).
Given the survival benefit of adding Herceptin to chemotherapy
in the advanced setting, several trials are ongoing to test Herceptin
in the adjuvant (node+) setting.
| Trial |
Schema |
NSABP
B-31 |
AC x 4 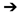 Paclitaxel x 4 vs.
Paclitaxel x 4 vs.
AC x 4  Paclitaxel x 4 plus Herceptin weekly x 1 year
Paclitaxel x 4 plus Herceptin weekly x 1 year |
NCCTG
9831/
Intergroup |
AC x 4 Paclitaxel
weekly x 12 vs. Paclitaxel
weekly x 12 vs.
AC x 4  Paclitaxel
weekly x 12 plus Herceptin weekly x 1 year Paclitaxel
weekly x 12 plus Herceptin weekly x 1 year
AC x 4 Paclitaxel
weekly x 12 Paclitaxel
weekly x 12  Herceptin
weekly x 1 year Herceptin
weekly x 1 year |
BCIRG
006 |
AC x 4  Docetaxel x 4 vs.
Docetaxel x 4 vs.
AC x 4  Docetaxel
x 4 plus Herceptin weekly x 1 year vs. Docetaxel
x 4 plus Herceptin weekly x 1 year vs.
Docetaxel + Carbo/cisplatin x 6 plus Herceptin weekly x 1 year |
CALGB
9804 |
Neoadjuvant AC +/- dexrazoxane 
Weekly paclitaxel x 12 +/- Herceptin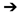
Surgery/radiation therapy +/-
Herceptin weekly x 1 year (2x2x2 design) +/-
Herceptin weekly x 1 year (2x2x2 design) |
| HERA |
Any chemo or XRT Observation
vs Herceptin q 3 wk x 12 mo vs Herceptin q 3 wk x 24 mo Observation
vs Herceptin q 3 wk x 12 mo vs Herceptin q 3 wk x 24 mo |
AC = doxorubicin 60 mg/m2 plus cyclophosphamide 600
mg/m2
Paclitaxel 175 mg/m2 q 3 wk unless specified weekly, then 90 mg/m2
Docetaxel 100 mg/m2 q 3 wk
Carbo/cisplatin - Carboplatin AUC 6 or cisplatin 75 mg/m2
q 3 wk
| A Visionary’s View of Breast Cancer
Management in the Next Decade Umberto Veronesi, MD, Scientific
Director, Istituto Europeo di Oncologia, Milano, Italy |
 |
A revolution in breast cancer treatment was ushered in the 1970s
with the conservative approach. After 25 years survival, curves
of the 701 cases enrolled in the Milan trial I show that quadrantectomy
gives identical results to the Halsted mastectomy.
A more recent development is the extension of quadrantectomy to
large tumors after initial (neo-adjuvant) chemotherapy to reduce
the size of the mass and permit a safely conservative approach.
One of the most exciting conservative approaches in breast cancer
surgery is sentinel node biopsy. In the first series of 376 patients
we identified the first node draining the tumor area with the aid
of the radiotracer (99Tc) and a gamma detecting used during surgery.
All patient underwent complete axillary dissection. The study showed
an overall accuracy of 96.8%, a sensitivity of 93.3% and a specificity
of 100%. We have now more than two thousand cases of sentinel node
biopsy including those in the randomized trial with the same accuracy
rate, with a mean follow up of 30 months and only 1 case in which
appeared axillary metastases.
By further exploiting the radiotracer and probe we developed a
new technique for precisely localizing non palpable lesions. Called
ROLL (radioguided occult lesion localization) we have used it on
more than 1000 patients to remove the lesion with the maximum precision
and accuracy as determine by intaoperative X-ray of the specimen.
Another important trial a the EIO recruited 436 patients with a
clinically negative axilla and a breast cancers of less than 1.2
cm who did not received axillary dissection, to assess the protection
afforded by prophylactic axillary irradiation. It was found that
axillary dissection may be avoid without significant risks in such
patients, as the 5-year survival was 98%.
We are now experimenting the intraoperative radiotherapy for limiting
the RT to the tumor bed giving the maximum dose of radiation at
the same time of operation without necessity of post operative therapy.
Also adjuvant treatments are reaching important goals by new and
more selective drugs thanks to the improvement in molecular targeting
with less side effects.
The new discipline of pharmacoprevention is emerging in breast
cancer. Tamoxifen is significantly protective in high risk women
but not in normal risk ones. HRT users may greatly benefit by tamoxifen
and a new trial is in progress at the European Institute of Oncology.
Finally, the use of vitamin A derivatives (fenretinide) can reduce
the risk of developing breast cancer in premenopausal women.
| The Use of the Decision Board for Breast
Cancer Treatment Dr. Tim Whelan, B.M., B.Ch., M.Sc., Associate
Professor, Dept. of Medicine and Director, Supportive Cancer
Care Research Unit, McMaster University, Ontario, Canada |
 |
In recent years there have been major advances in the treatment
of early stage breast cancer. The decisions a patient must make
about the treatment are often difficult and complex. In the past
physicians tended to make decisions for patients with little patient
input. Many patients now often express a desire for more information
about their disease and the need to be more actively involved in
the decisions about their treatment. Researches have responded by
investigating better ways of transferring information and involving
patients in decision making. Decision aids have been defined as,
“interventions designed to help people make specific and deliberative
choices among options by providing information on options and outcomes
relevant to a patient’s health”. The Decision Board consists
of a visual aid and written material. The instrument is administered
by a clinician during the patient consultation. Information from
clinical trials on a patient’s choices, outcomes, probabilities
of those outcomes and associated quality of life is presented in
an interactive step-by-step fashion.
The Decision Board was initially developed to help women with node
negative breast cancer to decide whether to receive adjuvant chemotherapy.
It was found to be both acceptable and helpful in decision making.
A cohort study of the Decision Board by patients deciding on radiation
therapy following lumpectomy found that the instrument improved
both patient knowledge and facilitated shared decision making. In
another study it was found that the use of the Decision Board for
women deciding between lumpectomy and mastectomy was well accepted
by both patients and surgeons in the community. Recently a randomised
trial of the Decision Board for chemotherapy in women with node
negative breast cancer demonstrated that the instrument improved
both patient knowledge and satisfaction with decision making. Currently
a number of studies are on going including a randomised trial to
evaluate the Decision Board process for women deciding on lumpectomy
and mastectomy and the development and testing of computer versions
of the Decision Board to facilitate the presentation of more complex
decisions to women with breast cancer. A more versatile decision
aid will respond to the diverse needs of patients and physicians
and may facilitate its wider use throughout the medical community.
| The Computer Program, Adjuvant!, A Tool
Assist In Adjuvant Therapy Decision Making for Early Breast
Cancer. Peter M. Ravdin, MD., Ph.D. , Associate Professor of
Medical Oncology, University of Texas, Health Science Center,
San Antonio, TX |
 |
The decision of whether a breast cancer should receive adjuvant
therapy involves weighing the risks and benefits of such therapy.
The most common presentation of breast cancer today is as Stage
1 disease with a low risk of relapse and breast cancer related death.
For many of these patients the benefit of adjuvant therapy is modest,
and its use somewhat controversial.
To address this problem various panels have drawn up treatment
guidelines. For example over the last year guidelines have been
published by an NCI Consensus Conference, the NCCN, and from St.
Gallen’s (an International Breast Cancer panel). A graphical
summary of these guidelines is shown below. What is interesting
about these recommendations is that there are a number of scenarios
where they reach quite different recommendations. Take the example
of patients with N0T1a estrogen receptor negative tumors. Treatment
recommendations would range from mandated adjuvant chemotherapy
(St. Gallen’s), to no therapy (NCCN).
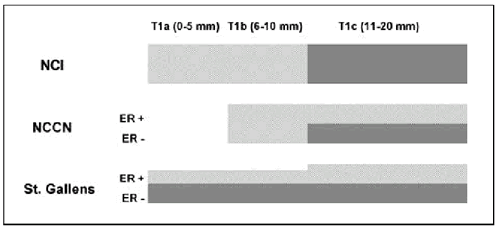
What is missing from these Guidelines is some quantitative sense
of what is gained or given up by getting selecting a given option.
A evidence based tool, a computer program, Adjuvant!, was created
to address this issue.
The steps to use the program, Adjuvant! are:
- Entering the usual patient and tumor related data. With the
program then projecting prognosis and competing mortality.
- Selecting the adjuvant therapy that is to be considered. With
Adjuvant! providing the efficacy estimates (proportional risk
reductions), and projecting probably net benefit.
- Printing out easy to read outcome sheets that might be used
in patient consultations.
- Printing out sheets with treatment schema and toxicity information.
Below is shown the Main Screen from Adjuvant!
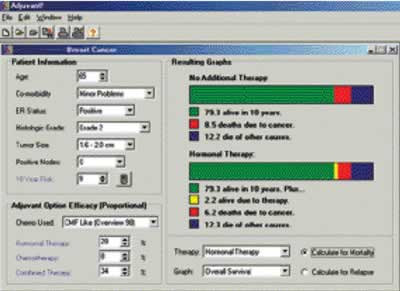
In the extensive help files there are discussions of topics of
special interest in adjuvant therapy, discussion of treatment guidelines,
and links to help in the consideration of clinical trials
Although guidelines help standardize therapy, tools such as Adjuvant!
have the power to better inform patients and make them true partners
in weighing treatment options.
- Adjuvant Therapy for Breast Cancer. NIH Consensus Statement
Online 2000 November 1-3; 17(4): 1-23.
- Carlson RW. Anderson BO. Bensinger W. Cox CE. Davidson NE.
Edge SB. Farrar WB. Goldstein LJ. Gradishar WJ. Lichter AS. McCormick
B. Nabell LM. Reed EC. Silver SM. Smith ML. Somlo G. Theriault
R. Ward JH. Winer EP. Wolff A. National Comprehensive Cancer Network.
NCCN Practice Guidelines for Breast Cancer. Oncology 14(11A):33-49,
2000 Nov.
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, and Senn H-J.
Meeting Highlights: International Consensus Panel on the Treatment
of Primary Breast Cancer. Journal of Clinical Oncology 19(18)
3817-3827, 2001.
- Ravdin PM. Siminoff L A. Davis GJ. Mercer MB. Hewlett J. Gerson
N. Parker HL. Computer program to assist in making decisions about
adjuvant therapy for women with early breast cancer. Journal of
Clinical Oncology. 19(4):980-91, 2001 Feb 15.
| Communicating therapeutic goals: Enlisting
the patient as partner Kathy D. Miller, M.D., Associate Professor
of Medicine, Indiana University, Indianapolis, IN |
 |
In newly diagnosed patients, the risk of recurrence and death differs
among patient subgroups, therefore the absolute reduction in relapse
and death rates will be greatest in high-risk patients and smallest
in low-risk patients. Because chemotherapy has toxicity, the art
of medicine involves balancing risk and benefit for individual patients.
With few exceptions, metastatic breast cancer is not curable making
the goal of ALL therapy palliative (as opposed to potentially curative),
requiring even greater patient inout into treatment decisions. Unfortunately
clinical trials, by their nature, attempt to simplify through the
rigorous application of study entry criteria. Consequently, virtually
every trial is necessarily unrepresentative of the general population
of breast cancer patients. In real life patients differ considerably
from each other, and these differences have real consequences for
the treating physician. These complexities include exposure to prior
therapy, presence of co-morbid conditions, psychosocial circumstances,
as well as emotional and spiritual needs. In real life clinical
therapy frequently requires a series of negotiations between patient
and physician; the patient’s needs and opinions definitely
matter. The wise physician is aware of an old truth: in the exam
room there are two experts, one an expert on the disease and the
other an expert on the patient.
| Research in complementary medicine Debu
Tripathy, M.D., UCSF Carol Franc Buck Breast Care Center, University
of California, San Francisco, CA |
 |
Complementary and alternative Medicine (CAM) has become increasing
popular, especially in patients with cancer. While some CAM modalities
have been practiced for centuries, very few studies have been done
to rigorously assess the safety and effectiveness of these approaches.
CAM includes many modalities - below is a partial list:
* Mind body medicine (meditation, yoga)
* Dietary approaches (macrobiotic diet, micronutrients)
* Herbal medicine
* Acupuncture
* Naturopathic medicine
* Homeopathy
* Reflexology
* Aromatherapy
Several reasons exist for the popularity of CAM in cancer:
1. Convention cancer treatment is only partially or minimally
effective in many settings. Most common epithelial (solid) tumors
in adults are not curable at the advanced stages. While many therapies
may induce transient responses, often time resistance will develop.
Furthermore, side effects of therapy can require discontinuation,
or in some cases, patient refusal of therapy altogether. Even newer
biologically targeted therapies tend to be effective only in a minority
of treated patients, and resistance to these novel agents eventually
develops as it does with conventional cancer drugs. Given the clear
limitations of standard cancer treatment, many patients with cancer
will opt to add CAM to their plan with the attitude that it cannot
hurt and might help.
2. CAM practitioners are often in a position to spend more time
with their patients and perhaps more time listening. This is in
contrast to conventional medicine where recent trends in managed
care and have forced physicians to spend less time with patients.
The rewards that patients receive in attentiveness make a difference
in their perception of trust and well being. This further popularizes
CAM and may even produce a favorable psychological and even health
benefit from using CAM. On the other hand, CAM usage may be a sign
of distrust of conventional medicine as well as a higher degree
of psychological stress and anxiety on the part of the patient.
3. Side effects of cancer and cancer treatment can have a significant
impact on quality of life. Many CAM modalities are geared to treating
symptoms and not necessarily the underlying disease. Conventional
palliative medicines such as narcotics and anti-emetics themselves
have undesirable side effects. Therefore, just as with therapy,
many individuals view CAM treatments as being more natural and holistic.
4. While most patients use CAM therapies along with conventional
treatment, many do not share this information with their physician.
Similarly, most physicians do not ask their patients about CAM usage
and are reluctant to discuss their views or opinions in this area.
Several reasons exist for the lack of the integration of CAM into
mainstream medicine. Most physicians lack training and exposure
to CAM modalities. Also, there has been a growing emphasis on evidence-based
medicine and very few CAM modalities have been subjected to rigorous
laboratory and clinical study. Hence, there is very little representation
of CAM in the medical literature, textbooks and scientific meetings.
All of these reasons can be considered valid from the standpoint
of patients’ attitudes and the frustrations of the limitations
in what can be done for some cancer patients. However, in many cases,
there is also potential merit in CAM and there is a growing recognition
that formal research of CAM needs to be performed in different cancer
settings. In 1993, the U.S. Congress mandated the opening of the
Office of Alternative Medicine in the National Institutes of Health.
Five CAM research centers were then funded by this Office. In 1998,
the Office was converted into the larger National Center for Complementary
and Alternative Medicine (NCCAM) and yearly funding is now in excess
of 60 million dollars a year. A special Office of CAM within the
National Cancer Center has also been established. These organizations
are actively releasing requests for applications in specific areas
of CAM and developing mechanisms and review teams to fund meritorious
work. There is a very slowly growing expertise of multidisciplinary
investigators cross-trained in clinical research and CAM practice.
Additionally, the National Cancer Institute has an information service
(PDQ) regarding clinical trials and disease-specific information.
Summaries about the background and scientific evidence for different
types of CAM is being generated and reviewed to made readily available
to the public. Surveys of both patients and physicians have demonstrated
an interest in CAM research and in particular, broad support for
clinical trials in this area.
In order to assess these therapies and begin to integrate them
into conventional cancer care, stepwise clinical trials will need
to be done in a similar fashion to the process that is needed for
currently available therapies and new experimental treatments. However,
several investigative challenges are posed by the nature of some
CAM approaches: These include:
* There are few preclinical (laboratory) models or well-documented
clinical series upon which to base or prioritize clinical trials.
* CAM approaches are often individualized, so applying the same
experimental variable to all subjects may not be in keeping with
the CAM modality. For example, herbal regimens are often complex
and highly individualized based not only on the disease but many
other characteristics of the patient.
* CAM therapies are usually geared towards many outcomes such
as tumor response, quality of life and emotional well being. From
a statistical standpoint, it is very difficult to demonstrate
efficacy in all of these endpoints.
* There is a lack of funding, cross-trained investigators and
qualified reviewers for research grants and publications
CAM research needs to follow the same basic principles of any biomedical
investigation. There should be a sufficient background and scientific
rationale for pursuing the treatment in question. The design of
the study must contain the same experimental and control groups
and must use standard statistical data analysis tools for definitive
conclusions to be made.
Laboratory methodology in CAM may not always apply given the nature
of some CAM approaches. For example, mind-body medicine is difficult
to model and study in the laboratory, although in human subjects,
laboratory studies can be done, for example assessing immune function
or cytokine release after prayer or meditation. In other cases,
laboratory studies are necessary prior to clinical investigation.
Herbal therapies can be tested against cancer cell lines if an anti-proliferative
effect is being sought. It is well recognized that these models
are not very accurate in predicting clinical success and may not
be able assess host-tumor interactions, but nevertheless, they can
serve a useful role in prioritizing and justifying clinical trials.
Our group has begun investigative work in CAM, using herbal therapy
as a model since there is a basis for the use of botanical products
in cancer. First, these are known to possess biological activity
and in fact, many modern drugs are derived from herbal components.
Second, there is some degree of consistency in the types of herbs
used for specific indications and there is also licensure and training
of herbalists in many states. Finally, herbal therapy represents
one of the more commonly used modalities for cancer. Below are several
studies in progress or completed by our group. In all cases, Investigation
New Drug Licenses (INDs) have been obtained from the FDA for these
IRB-approved studies.
- Combination Herbal Therapy to Alleviate Side Effects of Doxorubicin
and Cyclophosphamide Chemotherapy for Early Stage Breast Cancer.
A 21-herb combination that has been used by many practitioners
was formulated and standardized. This pilot study required a placebo
arm in order to differentiate potential herbal toxicities from
chemotherapy side effects and also to obtain an estimate of the
effect size in reducing side effects. Validated tools to assess
symptoms and quality of life are being used in this 60 patient
study
- Pilot Phase I Study of Tibetan Herbal Therapy for Advanced
Breast Cancer. This study was designed to preserve the individualization
of care by an expert Tibetan physician. Patients with minimally
symptomatic measurable advanced breast cancer were treated with
Tibetan herbal formula as their only treatment. The actual formula
was determined by the Tibetan physician based in his history and
examination. Standard safety assessments and tumor measurements
were performed and patients were treated until tumor progression.
No Grade III/IV toxicities were seen related to therapy and one
of 9 evaluable patients had a 4-month partial response.
- A Phase I/II study of Herba Scutellaria Barbatae (HSB) for
Advanced Breast Cancer. This study is similar to #2, but all patients
receive an extract of HSB, which emerged from our laboratory program
screening herbal extracts for antiproliferative activity on breast
cancer cell lines
| Breast Cancer Online: the internet as a
source of information for professionals J. F. R. Robertson,
Professor of Surgery, City Hospital, Nottingham, UK |
 |
There are several problems facing a researcher, clinician or patient,
in using the Internet to source information on breast cancer. Firstly
one must contend with the massive information overload that the
World Wide Web presents to the individual. This is compounded by
the lack of a proper, consistent peer-review process for material
made available over the Internet. It can therefore be extremely
dangerous to rely on data sourced purely off the net.
There are several strategies that one can adopt to search for material,
and the most common starting point is the large search engines,
such as Altavista or Google. The advantage of these search engines
is that they can conduct a comprehensive search across the Internet
for key phrases in seconds. However the disadvantages are often
evident when too many pages of dubious quality are returned. It
is possible to refine ones search and reduce the number of items
returned but as yet no method of verifying their quality exists.
A second option in commencing an Internet search is to use one
of the bibliographic databases, many of which are exclusively medical.
Leading examples include Medline and the cancer specific database
CancerLit. Such sources as entry points into online information
offer the user some confidence that the comprehensive search will
be limited to published material. Much of this will have undergone
the necessary process of peer-review as quality control. There still
remains one major disadvantage in that the user will often only
have access to the abstracts of material. Full-text papers, if available,
usually involve some kind of rights fee.
Another strategy would be to use one of the many news groups, which
are available to individuals with a specific interest. These are
essentially chat rooms. Within medicine, good examples are alt.support.cancer.breast
and sci.med.diseases.cancer. However once again the user cannot
be assured of the quality of the posted messages which may be open
to abuse and inaccuracy. On the other hand within specialist areas,
the members of the group will be known and their reputation acts
as an endorsement of quality. In a similar vein, new initiatives
based on the concept of the news group (such as clinical.netprints.org)
provide researchers with the opportunity to post research ahead
of publication whilst protecting copyright and inviting informal
peer-review. There are, however, currently no similar initiatives
specific to the breast cancer research community.
One of the most useful general methods for accessing information
on breast cancer from the web is via one of the online medical directories.
These are essentially compendiums of links to relevant web sites
and are a shortcut to the less specific Internet search engines.
Examples include Cancer Index and Medical Matrix. In these the user
can be confident that there has been a degree of quality control
of the links that are available. Additionally, these directories
list the web sites they include under specific categories and make
the task of identifying relevant sites much easier.
The last strategic option for the healthcare professional sourcing
information from the Internet is the specialist, peer-reviewed,
portal site. This combines the advantages of being comprehensive
in content and links with the quality control which is normally
only associated with the established print-on-paper journals. One
of these specialist sites is Breast Cancer Online (http://www.bco.org/)
that is specific to the needs of breast cancer professionals. It
contains peer-reviewed articles that are quality controlled by an
international editorial board, in much the same way as a traditional
peer-reviewed journal. Indeed, Breast Cancer Online and the more
research orientated Breast Cancer Research site, both have unique
identifiers, and can thus both be cited and indexed. The editorial
board also vets the links section and thus obviates the process
of assessment the user.
For the present, the best practical solution for the health care
professional seeking reliable information from the Internet on breast
cancer is to use a combination of the options described. To rely
on the general search engines is unwise and wasteful when compared
to the quality controlled medical directories or the peer-reviewed
portal sites, such as Breast Cancer Online. To search published
papers, the best of the bibliographic databases is PubMed but this
does exclude many journals (especially non-US ones) and it is not
always possible to access full text directly. For access to full-text
papers, the best source is to go to the relevant journal’s
homepage (for example, http://www.bmj.com/,
which is one of the best adaptations of a print on paper journal
to online) or the “clearing houses” for journal subsidiary
rights, such as High Wire or Catchword/Ingenta.
As the quantity of information on the Internet continues to grow
exponentially it seems the development of portal sites, such as
Breast Cancer Online, within all the medical subspecialties will
provide a pivotal role in allowing researchers and clinicians to
find their way through the jungle of information rapidly and with
confidence.
Back | Top of Page


|
|



