|
| |
|
| Breast Cancer Clinical Trials |
 |
| Adjuvant chemotherapy |
|
| |
|
 |
| PHASE III RANDOMIZED STUDY OF ADJUVANT
CHEMOTHERAPY USING STANDARD CYCLOPHOSPHAMIDE/ METHOTREXATE/FLUOROURACIL
(CMF) OR DOXORUBICIN/ CYCLOPHOSPHAMIDE (AC) VERSUS ORAL
CAPECITABINE IN ELDERLY WOMEN WITH OPERABLE ADENOCARCINOMA
OF THE BREAST (STUDY APPROVED, BUT TEMPORARILY CLOSED)
Open
Protocol |
|
| PROTOCOL IDS: |
CLB-49907, CTSU |
| PROJECTED ACCRUAL: |
A total of 600-1,800 patients (300-900 per treatment
arm) will be accrued for this study within 2-6 years . |
|

|
|
Beginning within 12 weeks after treatment in arm I or II,
patients with estrogen or progesterone receptor-positive disease
receive tamoxifen x 5 years.
Beginning 4-6 weeks after treatment in arm I or II, eligible
patients who previously underwent breast conservation surgery
undergo XRT.
Quality of life (QOL) is assessed at baseline; at 6 weeks,
9 weeks (ARM II), or 12 weeks; and then at 1, 12, 18, and
24 months after study.
Drug adherence is assessed at 9 weeks during study (ARM II).
Patients are followed every 6 months for 2 years and then
annually for 15 years.
|
 |
|
OBJECTIVES:
- Compare the effectiveness of adjuvant chemotherapy using
standard CMF or AC vs oral capecitabine in terms of disease-free
and overall survival in elderly women with
operable adenocarcinoma of the breast.
- Compare the quality of life and physical functioning
of patients treated with these regimens.
- Compare the toxicity of these regimens in these patients.
- Evaluate the adherence of older patients to an oral chemotherapy
regimen.
ELIGIBILITY
- Postmenopausal women age 65 and over
- Histologically proven operable adenocarcinoma of the breast
- Stage IIA or IIIA disease ( T1-3, N1, M0 or T2, N0, M0
if primary lesion at least 3 cm)
- Hormone receptor status not specified
- Must have undergone 1 of the following within the past
12 weeks: Modified radical mastectomy OR lumpectomy with
axillary lymph node dissection or sentinel node biopsy.
Prior full axillary dissection required if positive sentinel
node(s)
- Any number of previously excised nodes allowed
- No prior chemotherapy for breast cancer
- No other concurrent chemotherapy or hormonal therapy
STUDY CONTACT
Richard L. Schilsky, Chair Ph: 773-834-3914
Cancer and Leukemia Group B
|
 |
| STUDY OF TAMOXIFEN IN THE PREVENTION OF
SKELETAL AND CARDIOVASCULAR MORBIDITY OF ADJUVANT CHEMOTHERAPY
IN PREMENOPAUSAL WOMEN WITH STAGE I OR II BREAST CANCER
Open
Protocol |
|
| PROTOCOL IDS: |
NU-95B2; NCI-G00-1737 |
| PROJECTED ACCRUAL: |
A total of 80 patients (40 per stratum) will be accrued. |
|
| |
| |
 |
|
OBJECTIVES:
- Compare the bone density in the femoral neck and lumbar
spine and cholesterol levels in premenopausal women with
stage I or II breast cancer treated with adjuvant
chemotherapy with or without tamoxifen.
- Collect information regarding breast cancer risk factors,
treatment, pathology, diet, activity, weight and smoking.
PARTICIPATION CRITERIA
- Premenopausal women ages 30-50
- Histologically proven stage I or II breast cancer
- Scheduled to receive adjuvant chemotherapy +/- tamoxifen
PROTOCOL
Adjuvant chemotherapy +/- tamoxifen at the discretion of the
treating physician. Baseline levels of FSH, estradiol, total
cholesterol, HDL, LDL measured. Baseline bone densitometry
of the femoral neck and lumbar spine. Laboratory studies and
bone densitometry repeated at years 1 and 2.
STUDY CONTACT
Monica Morrow, Chair, Ph: 312-926-9039
Robert H Lurie Comprehensive Cancer Center
Northwestern University, Chicago, Illinois
|
 |
| PHASE III RANDOMIZED STUDY OF ZOLEDRONATE
AS ADJUVANT THERAPY IN PATIENTS WITH STAGE I, II OR IIIA
NONMETASTATIC BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
SWOG-S9905 |
| PROJECTED ACCRUAL: |
A total of 3,300 patients will be accrued for this study
over 3.5 years. |
|
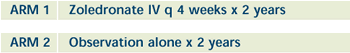 |
| |
 |
|
OBJECTIVES:
- Compare disease-free and overall survival in patients
with stage I, II or IIIA nonmetastatic breast cancer treated
with standard adjuvant therapy and zoledronate to those
treated with standard adjuvant therapy alone (obser vation
only).
- Assess whether zoledronate added to standard adjuvant
therapy influences the first site of recurrence in these
patients.
- Compare the first site of recurrence in PTHrP-positive
patients with the first site of recurrence in PTHrP-negative
patients in the group not receiving zoledronate.
- Explore whether treatment effects are different within
the PTHrP-positive and PTHrP-negative subsets.
PARTICIPATION CRITERIA
- Age, menopausal status, hormone receptor status: Not specified
- Histologically confirmed stage I, II or IIIA primary invasive
adenocarcinoma of the breast. No metastatic disease
- Must have undergone modified radical mastectomy or BCT
plus either ALND or SLNB
- Prior or concurrent standard systemic adjuvant therapy
required
- Prior neoadjuvant chemotherapy allowed
- Combined hormonal/chemotherapy or hormonal therapy alone
allowed
- Concurrent radiotherapy allowed
STUDY CONTACT
Julie R. Gralow, Chair, Ph: 206-288-1233
Southwest Oncology Group
|
 |
| PHASE III RANDOMIZED STUDY OF ADJUVANT
DOXORUBICIN AND CYCLOPHOSPHAMIDE FOLLOWED BY DOCETAXEL
VERSUS DOXORUBICIN AND DOCETAXEL VERSUS DOXORUBICIN, DOCETAXEL
AND CYCLOPHOSPHAMIDE IN WOMEN WITH BREAST CANCER AND POSITIVE
AXILLARY LYMPH NODES Open
Protocol |
|
| PROTOCOL IDS: |
NSABP B-30; CTSU |
| PROJECTED ACCRUAL: |
A total of 4,000 patients will be accrued within 3 years. |
|

|
T= Docetaxel A= Doxorubicin C= Cyclophosphamide
Tamoxifen given for 5 yrs to all ER+ or PR+ patients.
Tamoxifen given to ER- or PR- patients and receptor
unknown patients ³ 50 yrs old at physician’s direction. |
 |
|
OBJECTIVES:
- Compare the efficacy of adjuvant AC and docetaxel given
concurrently versus adjuvant AC followed by docetaxel, in
terms of overall survival and disease-free survival of women
with breast cancer and positive axillary lymph nodes.
- Compare the efficacy of adjuvant doxorubicin and docetaxel
versus regimens containing cyclophosphamide.
- Compare the toxic effects of these regimens.
- Compare the quality of life of these patients.
- Compare the differences in amenorrhea in premenopausal
women in each treatment arm and its relationship to symptoms,
quality of life, disease-free survival and overall survival.
PARTICIPATION CRITERIA
- Age unspecified, hormone receptor status known
- Histologically proven invasive adenocarcinoma of the breast
confined to the breast and ipsilateral axilla on clinical
exam
- Stage I, II or IIIA (T1-3, N0-1, M0)
- At least one axillary lymph node with tumor on histologic
exam
- Sentinel node biopsy allowed if followed by axillary dissection
- No suspicious palpable nodes in the contralateral axilla
or palpable supraclavicular or infraclavicular nodes, unless
proven on biopsy to not be involved with tumor
- No bilateral malignancy or mass in the opposite breast,
unless mass is histologically proven to be benign
- Must have undergone either a prior total mastectomy and
axillary dissection (modified radical mastectomy) OR prior
lumpectomy and axillary dissection
- Patients must receive radiotherapy after randomization
- Margins must be clear
- No N2 disease and/or any positive nonaxillary lymph nodes
- No metastatic disease by X-ray, MRI or biopsy
- Skeletal pain allowed if bone scan negative for metastases
STUDY CONTACT
Sandra M Swain, Chair, Ph: 301-435-9039
National Surgical Adjuvant Breast and Bowel Project
|
 |
| PHASE III RANDOMIZED STUDY OF ZOLEDRONATE,
CALCIUM AND CHOLECALCIFEROL (VITAMIN D) TO PREVENT BONE
LOSS IN WOMEN WITH BREAST CANCER RECEIVING ADJUVANT CHEMOTHERAPY
Open
Protocol |
|
| PROTOCOL IDS: |
CLB-79809, NCI-P01-0184 |
| PROJECTED ACCRUAL: |
Approximately 400 patients (200 per treatment arm) will
be accrued for this study within 24 months. |
|

|
| |
 |
|
OBJECTIVES:
- Compare the bone mineral density in the lumbar spine
after 12 and 36 months of therapy with zoledronate, calcium,
and cholecalciferol (vitamin D) in women with breast cancer
receiving adjuvant chemotherapy.
PARTICIPATION CRITERIA
- Age: 40 and over
- Premenopausal
- Histologically confirmed adenocarcinoma of the breast
by fine needle aspirate, biopsy (tru-cut, core, stereotactic),
lumpectomy or modified radical mastectomy
- Stage I-III (any T, any N, M0)
- Stage IV due solely to supraclavicular node involvement
allowed
- Plan to use adjuvant chemotherapy with or without tamoxifen
STUDY CONTACT
Charles L. Shapiro, Chair Ph: 614-293-7530
Cancer and Leukemia Group B
|
 |
| PHASE III STUDY OF ADJUVANT EPIRUBICIN
WITH OR WITHOUT DOCETAXEL AND CONCURRENT OR SEQUENTIAL
TAMOXIFEN IN POSTMENOPAUSAL WOMEN WITH NODE-POSITIVE BREAST
CANCER Open
Protocol |
|
| PROTOCOL IDS: |
ICCG-C/14/96, EU-20040 |
| PROJECTED ACCRUAL: |
A total of 800 patients will be accrued for this study. |
|

|
| Patients may be further randomized to receive
tamoxifen x 5 years concurrently with adjuvant chemotherapy
or sequentially after completion of chemotherapy. |
 |
|
OBJECTIVES:
- Compare the impact of adjuvant epirubicin with or without
docetaxel and concurrent or sequential tamoxifen on time
to relapse and overall survival in postmenopausal women
with node-positive breast cancer.
- Compare the toxic effects of these regimens in this patient
population.
- Compare the quality of life in terms of shift in long-term
toxicity and differences in recuperation.
- Compare the incidence of thromboembolic events during
the first 9 months of study and the influence of such events
on compliance in women treated with these regimens.
PARTICIPATION CRITERIA
- Histologically proven node-positive breast cancer
- Postmenopausal
- No distant metastases
STUDY CONTACT
Raoul C Coombes, Chair, Ph: +44 (0)20 8846 14 18
International Collaborative Cancer Group
Charing Cross Hospital
London, England, United Kingdom
|
 |
| PHASE III RANDOMIZED STUDY OF ADJUVANT
CLODRONATE WITH OR WITHOUT SYSTEMIC CHEMOTHERAPY AND/OR
TAMOXIFEN IN WOMEN WITH EARLY-STAGE BREAST CANCER Open
Protocol |
|
| PROTOCOL IDS: |
NSABP-B-34, CTSU |
| PROJECTED ACCRUAL: |
A total of 2,400 patients will be accrued for this study
within 4 years. |
|

|
| Adjuvant systemic therapy including tamoxifen
at investigator’s discretion |
 |
|
OBJECTIVES:
- Determine whether clodronate administered alone or in
addition to adjuvant chemotherapy and/or tamoxifen improves
disease-free survival in patients with early-stage breast
cancer.
- Determine whether clodronate reduces the incidence of
skeletal metastases and nonskeletal metastases.
- Determine whether clodronate improves overall and relapse-free
survival in these patients.
- Determine whether clodronate reduces the incidence of
skeletal morbidity (e.g., skeletal fractures, hypercalcemia,
skeletal pain, need for radiotherapy, spinal cord
compression) in these patients.
- Determine the relevance of serum markers of bone turnover
as a prognostic factor for the development of bone metastases
in these patients.
PARTICIPATION CRITERIA
- Histologically proven invasive adenocarcinoma of the breast
Stage I or II (T1-3, N0-1, M0)
- No significant nonmalignant bone disease that is likely
to interfere with interpretation of bone X-rays
- Skeletal pain allowed only if bone scan and/or roentgenological
examination fails to disclose metastatic disease
- Suspicious findings must be confirmed as benign by X-ray,
MRI or biopsy
STUDY CONTACT
Alexander H. G. Paterson, Chair, Ph: 403-670-1707
National Surgical Adjuvant Breast and Bowel Project
|
 |
| PHASE III RANDOMIZED STUDY OF ADJUVA N
T CYC LOPHOSPHAMIDE, EPIRUBICIN AND FLUOROURACIL VERSUS
CYCLOPHOSPHAMIDE, EPIRUBICIN, FILGRASTIM (G-CSF) AND EPOETIN
ALFA FOLLOWED BY PACLITAXEL VERSUS CYCLOPHOSPHAMIDE AND
DOXORUBICIN FOLLOWED BY PACLITAXEL IN PREMENOPAUSAL OR
EARLY POSTMENOPAUSAL WOMEN WITH PREVIOUSLY RESECTED NODE-POSITIVE
OR HIGH-RISK NODE-NEGATIVE STAGE I-IIIA BREAST CANCER
Open
Protocol |
|
| PROTOCOL IDS: |
CAN-NCIC-MA21, AMGEN-CAN-NCIC-MA21, BMS-CAN-NCIC-MA21,
JANSSEN-CAN-NCIC-MA21, P-UPJOHN-CAN-NCIC-MA21 |
| PROJECTED ACCRUAL: |
A total of 1,500 patients (500 per treatment arm) will
be accrued for this study within 3 years. |
|

|
T=paclitaxel
All arms: Treatment continues in the absence of disease progression
or unacceptable toxicity. |
 |
|
OBJECTIVES:
- Compare the disease-free survival and overall survival
of premenopausal or early postmenopausal women with previously
resected node-positive or high-risk node-negative stage
I-IIIA breast cancer treated with cyclophosphamide, epirubicin
and fluorouracil vs cyclophosphamide, epirubicin, filgrastim
(G-CSF) and epoetin alfa followed by paclitaxel vs cyclophosphamide
and doxorubicin followed by paclitaxel.
- Compare the rate of toxic effects of these regimens.
III. Compare the quality of life of patients treated with
these regimens.
PARTICIPATION CRITERIA
- Age: 60 and under
- Histologically confirmed adenocarcinoma of the breast
that is potentially curable
- Stage I-IIIA (T0-4 (dermal involvement only), N0-2, M0)
- Axillary node-positive or high-risk node-negative
- Previously treated with total mastectomy and axillary
node dissection or partial mastectomy and axillary node
dissection with planned breast radiotherapy after completion
of study or sentinel node biopsy (if node-positive, must
undergo axillary node dissection)
- No prior immunotherapy, chemotherapy, radiotherapy or
hormonal therapy for breast cancer
STUDY CONTACT
Margot J Burnell, Chair, Ph: 506-648-6884
NCIC-Clinical Trials Group
|
 |
| COMPANION STUDY TO EVALUATE L ATE CARDIAC
EFFECTS IN WOMEN WITH NODE-NEGATIVE BREAST CANCER RECEIVING
ADJUVANT CHEMOTHERAPY ON PROTOCOL WOG-8897 Open
Protocol |
|
| PROTOCOL IDS: |
SWOG-9342 |
| PROJECTED ACCRUAL: |
A total of 420 patients will be accrued for this study.
After initial accrual is completed, approximately 50 additional
patients will be accrued at 10 years. |
|
| |
| |
 |
|
OBJECTIVES:
- Compare the frequency of subclinical congestive heart
failure by measuring resting MUGA at 5-8 and 10-11 years
after randomization in women receiving adjuvant chemotherapy
with cyclophosphamide, methotrexate and fluorouracil or
cyclophosphamide, doxorubicin and fluorouracil on protocol
SWOG-8897.
- Estimate the frequency of late cardiac effects (congestive
heart failure, cardiac ischemic events and clinical symptoms)
in these patients treated with these regimens.
- Monitor prospectively the incidence of annual cardiac
events between the fifth and tenth year after randomization
of these patients to these regimens.
PARTICIPATION CRITERIA
- Age: 18 and over
- Women registered on Arm I, II, III or IV of protocol SWOG-8897
who have completed at least 1 course of assigned chemotherapy
- Completion of tamoxifen therapy not required
- Registration to current study required between 5.25-8
years or 10-11 years after randomization to protocol SWOG-8897
- Patients must be diagnosed disease-free with no prior
recurrence after registration on protocol SWOG-8897
PROTOCOL
The treating physician completes patient cardiovascular and
routine history and physical examination questionnaires at
baseline and yearly. Patients undergo resting MUGA scans
at 5-8 and 10-11 years after registration on protocol SWOG-8897.
The first scan must be performed within 3 months prior to
enrollment or within 1 month after registration on the current
study, and the second scan must be done in the tenth year
of follow-up and within 3 months prior to enrollment or 1
month from the anniversary of registration on the current
study.
STUDY CONTACT
Patricia A Ganz, Chair, Ph: 310-206-1404
Southwest Oncology Group
|
 |
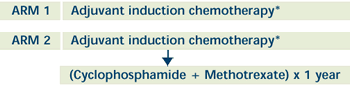
| PHASE III RANDOMIZED STUDY OF ADJUVANT
INDUCTION CHEMOTHERAPY WITH OR WITHOUT CYCLOPHOSPHAMIDE
AND METHOTREXATE AS MAINTENANCE CHEMOTHERAPY IN PATIENTS
WITH STAGE I, II, OR III BREAST CANCER
Open Protocol |
|
| PROTOCOL IDS: |
IBCSG-22-00, EU-20119 |
| PROJECTED ACCRUAL: |
Approximately 1,330 patients will be accrued for this
study within 5 years. |
|
|
|
*Patients received one of the following adjuvant induction
chemotherapy regimens:
AC, EC, CMF or AC or EC —> CMF or CEF or CAF
Patients with BCT receive XRT following completion of induction
chemotherapy.
|
 |
|
OBJECTIVES:
- Determine the efficacy of adjuvant induction chemotherapy
with or without cyclophosphamide and methotrexate as maintenance
chemotherapy in patients with stage I, II or III breast
cancer.
- Compare the disease-free, overall and systemic disease-free
survival of patients treated with these regimens.
- Compare the toxic effects of these regimens in these
patients.
- Compare the quality of life of patients treated with
these regimens.
PARTICIPATION CRITERIA
- Pre-or postmenopausal
- Histologically confirmed stage I, II or III breast cancer
(T1-3, N0-1, M0. T4 disease with minimal dermal invasion
allowed)
- No distant metastases
- Prior mastectomy OR BCT within past 6 weeks
- Estrogen and progesterone receptor-negative
- No prior radiotherapy for breast cancer
STUDY CONTACT
Marco Colleoni, Chair, Ph: 039-2-57489439
International Breast Cancer Study Group
|
PHASE II STUDY OF ADJUVANT DOSE INTENSIVE, SEQUENTIAL
CHEMOTHERAPY WITH DOXORUBICIN, PACLITAXEL, AND CYCLOPHOSPHAMIDE
FOR RESECTED STAGE II/III BREAST CANCER Open
Protocol
PROTOCOL IDS: YALE-HIC-7374; NCI-V95-0720
STUDY CONTACT:
Barbara A. Burtness, Ph: 203-785-6007
Yale Comprehensive Cancer Center
New Haven, Connecticut

PHASE III RANDOMIZED STUDY OF ADJUVANT DOCETAXEL
AND DOXORUBICIN VERSUS DOXORUBICIN WITH OR WITHOUT CYCLOPHOSPHAMIDE,
FOLLOWED BY COMBINATION CHEMOTHERAPY IN WOMEN WITH NODE POSITIVE
BREAST CANCER Open
Protocol
PROTOCOL IDS: BIG-2-98; EU-20002;
RP-56976-V-315
STUDY CONTACT:
Martine J. Piccart-Gebhart, Ph: 32-2-5413206
Institut Jules Bordet
Brussels (Bruxelles), Belgium

PHASE III RANDOMIZED STUDY OF CHEMOTHERAPY AND SURGERY
COMPARING ADJUVANT DOXORUBICIN FOLLOWED BY CMF (CYCLOPHOSPHAMIDE,
METHOTREXATE, AND FLUOROURACIL) VERSUS ADJUVANT DOXORUBICIN AND
PACLITAXEL FOLLOWED BY CMF VERSUS PRIMARY DOXORUBICIN AND PACLITAXEL
FOLLOWED BY CMF IN WOMEN WITH OPERABLE BREAST CANCER AND TUMOR GREATER
THAN 2 CENTIMETERS Open
Protocol
PROTOCOL IDS: INT-23/96; EU-97001
STUDY CONTACT:
Gianni Bonadonna, Ph: 2-70638485
Instituto Nazionale per lo Studio e la Cura dei Tumori
Milano (Milan), Italy

PHASE I/II RANDOMIZED STUDY OF ADJUVANT DOXORUBICIN
AND CYCLOPHOSPHAMIDE WITH OR WITHOUT CHINESE HERBAL THERAPY FOR
SYMPTOM MANAGEMENT IN WOMEN WITH STAGE I, II, OR EARLY STAGE III
BREAST CANCER Open
Protocol
PROTOCOL IDS: UCSF-CRO-97755, NCI-G01-2042,
UCSF-IND-54870
STUDY CONTACT:
Debashish Tripathy, Ph: 415-3537618
UCSF Cancer Center and Cancer Research Institute
San Francisco, California, U.S.A

PHASE III RANDOMIZED STUDY OF ADJUVANT CYCLOPHOS-PHAMIDE,
METHOTREXATE, AND FLUOROURACIL WITH OR WITHOUT EPIRUBICIN IN WOMEN
WITH EARLY STAGE BREAST CANCER Open
Protocol
PROTOCOL IDS: CRC-TU-NEAT; EU-98041
STUDY CONTACT:
Helena Earl, Ph: 01223-274312
Addenbrooke’s NHS Trust
Cambridge, England, United Kingdom

PHASE II STUDY OF AUTOLOGOUS HEAT SHOCK PROTEIN 70
VACCINE IN PATIENTS WITH HIGH-RISK BREAST CANCER Open
Protocol
PROTOCOL IDS: UCHC-01062, UCHC-1345-01
STUDY CONTACT:
Zihai Li, Chair Ph: 860-679-7979
University of Connecticut Health Center

Back | Top of Page

|
|



