|

What is the optimal adjuvant endocrine therapy
for premenopausal women with invasive breast cancer, including the
role of ovarian ablation/suppression?
 |
|
OVERVIEW:
The International Breast Cancer Overview has clearly
demonstrated that adjuvant ovarian ablation significantly
reduces mortality in premenopausal women, and tamoxifen
reduces mortality in both pre- and postmenopausal women
with ER-positive tumors. Current clinical trials are
addressing a number of important issues related to these
two key interventions, including the impact of combining
these therapies with chemotherapy. Trials are also underway
combining anastrozole with ovarian suppression in premenopausal
women, an interesting combination in light of the recent
ATAC outcome data.
|
|
In addition to chemotherapy, which adjuvant endocrine
therapy, if any, would you recommend for the following 43-year-old
premenopausal women with ER-positive, HER2-negative breast cancer?
|
TUMOR SIZE/
NODAL STATUS
|
|
TAMOXIFEN
|
ANASTROZOLE*
|
LETROZOLE*
|
OVARIAN
SUPPRESSION/
ABLATION
|
|
2.2 cm tumor
10+ nodes
|
80%
|
5%
|
5%
|
10%
|
|
2.2 cm tumor
2+ nodes
|
80%
|
5%
|
5%
|
10%
|
|
2.2 cm tumor
neg nodes
|
75%
|
15%
|
5%
|
5%
|
|
0.8 cm tumor
neg nodes
|
80%
|
5%
|
5%
|
10%
|
Which adjuvant endocrine therapy, if any, would
you recommend for the following 43-year-old premenopausal women
with ER-positive, HER2-positive breast cancer?
|
TUMOR SIZE/
NODAL STATUS
|
|
TAMOXIFEN
|
ANASTROZOLE*
|
LETROZOLE*
|
OVARIAN SUPPRESSION/
ABLATION
|
NONE
|
|
2.2 cm tumor
10+ nodes
|
80%
|
10%
|
5%
|
5%
|
-
|
|
0.8 cm tumor
2+ nodes
neg nodes
|
70%
|
10%
|
5%
|
10%
|
5%
|
*Aromatase inhibitors are not recommended to
premenopausal women without ovarian suppression.
| How many women have you treated with
both an aromatase inhibitor and an LHRH agonist or other
form of ovarian ablation for metastatic breast cancer? |
|
|
HISTORY OF OVARIAN ABLATION
It is just over 100 years since George Beatson described the dramatic
responses of three women with advanced breast cancer to surgical
cast ration. This became standard treatment for premenopausal women
with advanced breast cancer, and provided the first approach to
trials of adjuvant systemic thera py by pioneers Dr Cole (Manchester
) and Dr Nissen-Meyer (Oslo). Unfortunately, they were underpowered
statistically, and although they suggested that relapse-free survival
could be prolonged, it was not until the first world overview that
we could confidently conclude that such an approach improved survival.
—Michael Baum, ChM, FRCS;
Joan Houghton, BSc
Br Med J 1999;319:568-571.
OVERVIEW DATA ON OVARIAN ABLATION
Since the ove rview data is from older studies, the number of
patients in the ovarian ablation trials are few compared to the
number in t a m oxifen or chemothera py trials. There were several
thousand patients in the 1960s and 1970s, randomized to ovarian
ablation or observation, at first — mainly in the absence of
any chemothera py. In some of the later trials, chemothera py plus
ovarian ablation versus the same chemothera py alone was studied.
In fact, there are very clear data that ovarian ablation is beneficial
compared to no systemic treatment, with improvement much like what
was seen with the chemotherapy regimens of that day: CMF, AC, etc.
Because the studies are so old, the receptor status wasn't known
in many of the patients. In the later trials, ovarian ablation added
to chemotherapy isn't quite as significantly effective, although
it tends to add something to chemothera py. It may be that some
of these patients become amenorrheic with chemothera py, so that
adding ovarian ablation doesn't do as much.
—Kathleen Pritchard, MD
INTERGROUP 0101 STUDY OF ADJUVANT
OVARIAN ABLATION
The study was designed a long time ago, at a time when there was
increasing interest in ovarian ablation, because of the meta-analysis
and the availability of drugs that could lead to chemical castration
as opposed to surgical ablation.
In the best of all worlds, we would have had a 4th arm of CAF
followed by tamoxifen. At the time, however, we weren't sure that
we could pull it off statistically — that we could accrue to
that trial in a timely fashion and have something to talk about.
The disease-free survival was better for the group receiving CAFZT
compared to CAFZ. There was a borderline improvement with CAFZ compared
to CAF. An unplanned preliminary, retrospective subset analysis
demonstrated that younger women — arbitrarily defined as women
under the age of 40 — seemed to do better with goserelin. Perhaps
that's not surprising, because those women are the least likely
to be made postmenopausal by chemotherapy. There's also a suggestion
that women with premenopausal estrogen levels after chemotherapy
were destined to derive benefit from goserelin. The big clinical
question now is what to do with the young woman who is premenopausal
at the end of chemotherapy? Most of them receive tamoxifen as a
matter of routine. I personally am not using LHRH agonists in that
situation right now, but some of our very good colleagues have looked
at these trial results and said that they think it is legitimate
to do.
—Nancy E Davidson, MD
ADJUVANT OVARIAN ABLATION IN A NONPROTOCOL SETTING
We now have several trials demonstrating that, in receptor-positive
premenopausal patients, ovarian ablation is as effective as CMF.
In fact, there's almost a suggestion that it is better. In the ECOG
study (Intergroup 0101), patients received CAF, which everyone would
consider state-of-the-art chemotherapy. In that trial, there was
additional benefit from adding ovarian ablation to CAF — certainly
among women under age 40. That study really changed my thinking.
If a woman receives adjuvant chemotherapy and does not stop menstruating,
I routinely add the LHRH agonist, goserelin with tamoxifen.
—I Craig Henderson, MD
COMBINATION ENDOCRINE THERAPY
Combination endocrine therapy is a conceptual change for us. We
eliminated that approach a couple of decades ago. Perhaps because
of small, inadequately powered trials that were unable to detect
the type of differences we can identify today with larger studies.
Combination endocrine therapy may be more effective than single
agent treatment. With better tools now available, we are returning
to the concept of complete estrogen blockade — a strategy that
started several decades ago with hypophysectomy and adrenalectomy.
Studies of chemical ovarian suppression plus tamoxifen suggest greater
efficacy than either alone.
—Gabriel Hortobagyi, MD
STUDY OF GOSERELIN PLUS ANASTROZOLE IN METASTATIC
DISEASE
Endocrine maneuvers are often limited in premenopausal women,
however, we know that premenopausal patients with ER-positive tumors
are just as likely to be sensitive to hormone therapy as postmenopausal
women. We decided that, for patients on tamoxifen and goserelin,
there was no reason why we shouldn't treat them in the same way
as we would a postmenopausal woman who progresses on tamoxifen.
You stop the tamoxifen and start an aromatase inhibitor. We had
a series of women where that's what we did. We stopped the tamoxifen,
continued the goserelin, and then substituted anastrozole for the
tamoxifen. A significant percentage of those women responded.
—John F Robertson, MD, FRCS
AUSTRIAN TRIAL
A trial has been started in Austria that looks at goserelin plus
anastrozole as an adjuvant therapy. The key randomization is to
goserelin plus tamoxifen or goserelin plus anastrozole. The basis
for that is there was a study presented at ASCO, whereby patients
were randomized either to CMF or to goserelin plus tamoxifen.
This was in premenopausal, ER-positive patients. It showed that
the early recurrence-free survival curves were in favor of the endocrine
arm. Therefore they have taken that as the control arm for the next
study. They're now comparing goserelin plus tamoxifen to goserelin
plus anastrozole.
—John F Robertson, MD, FRCS
AROMATASE INHIBITORS IN WOMEN MADE MENOPAUSAL
BY LHRH AGONISTS
There is one limited metastatic study in ER-positive patients
demonstrating that goserelin plus anastrozole yielded similar responses
to what has been seen in postmenopausal patients. We need to design
appropriate trials to address this issue. It is very important to
consider that aromatase inhibitors as monotherapy should not be
used in premenopausal patients. But it would be a very interesting
and logical approach to design trials of complete estrogen blockade
in ER-positive premenopausal patients, using an LHRH agonist and
anastrozole.
—Aman Buzdar, MD
 |
| ABCSG-12: OVARIAN SUPPRESSION PLUS ANASTROZOLE
OR TAMOXIFEN IN HORMONE RECEPTOR-POSITIVE, NODE-NEGATIVE
OR NODE-POSITIVE BREAST CANCER No Protocol Link |
|
| PROJECTED ACCRUAL: |
1,250 patients will be accrued to this study. |
|
|
|
| All therapy given for 3 years. |
 |
|
STUDY CONTACT:
Raimund Jakesz, Chair
Austrian Breast & Colon Cancer Study Group
Vienna, Austria
|
 |
| A PHASE II TRIAL OF ANASTROZOLE PLUS GOSERELIN
IN THE TREATMENT OF HORMONE RECEPTOR-POSITIVE, METASTATIC
CARCINOMA OF THE BREAST IN PREMENOPAUSAL WOMEN No Protocol
Link |
|
| PROTOCOL: |
Goserelin q 4 weeks + anastrazole q day until disease
progression. |
|
| |
| Anastrazole starts 3 weeks after the initial
administration of goserelin. |
 |
|
STUDY CONTACT:
Robert W. Carlson, MD
Christine Schurman, RN
650-723-8686
|
 |
| EST-5188, INT-0101: PHASE III RANDOMIZED
COMPARISON OF ADJUVANT THERAPIES IN PREMENOPAUSAL WOMEN
WITH RESECTED NODE-POSITIVE HORMONE RECEPTOR-POSITIVE
ADENOCARCINOMA OF THE BREAST CLOSED
PROTOCOL |
|
| PROJECTED ACCRUAL: |
1,537 patients we re entered into the trial. |
|
|
|
CAF=cyclophosphamide, doxorubicin, fluorouracil;
Z=goserelin; T=tamoxifen |
 |
 |
| INTERGROUP 0101: DISEASE-FREE AND OVERALL
SURVIVAL |
|
| |
7 year
Disease-free Survival
|
7 year
Survival
|
|
|
| CAF |
58%
|
77%
|
|
|
| CAF/Zoladex |
64%
|
78%
|
|
|
| CAF/Zoladex/Tamoxifen
|
73%
|
80%
|
| |
|
|
| Davidson NE. 2000 NIH Consensus
Development Conference on Adjuvant Therapy for Breast
Cancer; Abstract |
|
| |
 |
| |
| INTERNATIONAL
OVERVIEW RESULTS OF ADJUVANT OVARIAN ABLATION, TAMOXIFEN AND
CHEMOTHERAPY |
 |
Adapted from Lancet 1996;348:1189-1196. Abstract
Lancet 1998;353:930-942. Abstract |
|
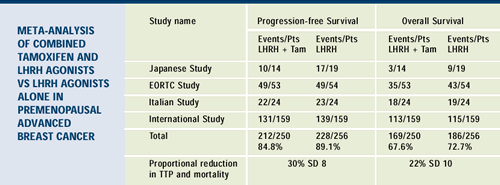
Derived from Klijn JGM et al. Combined tamoxifen and luteinizing
hormone-releasing hormone (LHRH) agonist versus LHRH agonist
alone in premenopausal advanced breast
cancer: A meta-analysis of four randomized trials. J Clin
Oncol 2001;19(2):343-353. Abstract
|
View References
Back | Top of Page

|
|



