|

What is the optimal algorithm for assessment of tumor
HER2 status?
 |
|
OVERVIEW:
Approximately 20-30% of women with breast cancer express
HER2 gene amplification, which along with protein overexpression
are strong prognostic factors and predictors of response
to trastuzumab. HER2 status determination is an important
component in the diagnostic evaluation of a patient
with breast cancer. The various methods for determining
HER2 status measure gene amplification, RNA overexpression,
or protein overexpression. HER2 protein overexpression
is frequently measured by immunohistochemistry (IHC),
while fluorescence in situ hybridization (FISH) detects
HER2 gene amplification. Results obtained with IHC and
FISH are not always concordant. The clinical outcomes
associated with trastuzumab are dependent upon the HER2
status of the patient. Although patients with 2+ HER2
protein overexpression do experience benefits, the response
rates for trastuzumab, as a single agent and in combination
with chemotherapy, are greater for patients with 3+
HER2 protein overexpression. Retrospective analyses
demonstrate that patients with HER2 gene amplification
are more likely to benefit from trastuzumab with respect
to overall response and survival. Since the scientific
literature on the subject of HER2 is still evolving,
clinicians are advised to follow this topic closely
as it continues to develop.
|
|
 |
 |
| |
|
|
In
approximately what percentage of your patients with
primary breast cancer do you assess HER2 status? |
100%
|
In
the last year, approximately how many women have you
evaluated and/or treated with metastatic breast cancer? |
Median = 53
|
In
approximately what percentage of your patients
with metastatic breast cancer do you attempt to
assess HER2 status? |
100%
|
| About
how many patients were HER2-positive? |
Median = 10
|
| How often do you obtain FISH
results on a patient you are treating? |
|
|
 |
| In approximately how many patients
have you asked a lab to do FISH testing? |
|
|
DISCORDANCE IN THE ASSESSMENT OF HER2 STATUS
There is a significant problem with HER2 testing in the community.
When we designed our adjuvant study — evaluating trastuzumab
in combination with chemotherapy — we included a plan for central
analysis of HER2 status. Unfortunately, when we looked at the initial
119 patients, we found a high level of discordance in HER2 testing
by IHC, and even by FISH, when comparing measurements in the community
versus central testing.
We found discordant results in six of the nine FISH test assays.
These were FISH-positive in the community, but FISH-negative in
the central lab. The number of patients is very, very small to date,
so we cannot conclude that FISH is a bad test to be performed in
the community, but we need to look into why this discordance occurs.
IHC concordance between the community and central laboratories was
about 75 percent.
We've done another study of HER2 testing, based on 1,500 specimens
sent to Mayo medical laboratories over a five-month period. We took
213 specimens labeled as HER2 2+ and evaluated them for protein
overexpression and gene amplification, and we found that only 12
percent of the tumors scored as 2+ by the HercepTest™ actually
were FISH-positive.
—Edith A Perez, MD
ASSESSING HER2 STATUS
The NCCN guidelines call for HER2 testing of all breast cancers;
however, this year we were much more specific than in the past.
We call for HER2 testing by IHC, and if the IHC result is 2+ by
the HercepTest™, we call for FISH analysis. This is primarily
because in the metastatic setting, when you're looking for benefit
or lack thereof from trastuzumab, FISH-positivity is by far the
best predictor of responsiveness.
Women whose breast cancers are IHC 3+ by the HercepTest™
are almost all FISH-positive, while those that are IHC 0 or 1+ are
almost always FISH-negative. This approach is based upon the study
reported by Chuck Vogel, which looked at trastuzumab as a single
agent and found very good rates and long duration of response in
those women who were either IHC 3+ or IHC 2+ and FISH-positive.
—Robert W Carlson, MD
HER2 ASSESSMENT
Reliable detection of HER2 overexpression is important for the
success of trastuzumab (Herceptin®) therapy. Several methods
are available for measuring HER2 expression at the DNA, RNA or protein
level. The method most frequently employed is immunohistochemical
(IHC) detection of the HER2 receptor in paraffin sections. Advantages
include the precise localization of the HER2 protein, the availability
of paraffin material and the ease of the procedure.
However, IHC can be influenced by the sensitivity/specificity
of the antibody, tissue treatment and, in particular, subjective
assessment. These disadvantages do not exist in the detection of
gene amplification by fluorescence in situ hybridization (FISH)
or polymerase chain reaction. However, FISH requires expensive equipment
that is not widely available in pathology laboratories. Another
approach quantitates shed HER2 antigen in the serum by an enzyme-linked
immunosorbent assay.
The key advantage of this method is the ease of sampling blood,
however, serum HER2 concentrations do not accurately reflect the
tumor status. Furthermore, this method does not register single-cell
expression, which is important for therapeutic decision-making.
For routine diagnostics, the combination of IHC and FISH is useful.
In addition, to improving the accuracy and comparability of HER2
assays, these optimized protocols may further enhance the efficacy
of t rastuzumab thera py by selecting those patients most likely
to respond.
—Gerhard Schaller, MD et
al.
Ann Oncol 2001;(Suppl 1):S97-S100.
HER2 REPRODUCIBILITY
Consensus regarding the best methods, reagents, or cut-off points
to define HER2 status for determining trastuzumab responsivity has
not yet been reached. HER2 testing for other prognostic or predictive
purposes, e.g. to determine whether patients are likely to respond
to other agents, such as dose-intensive doxorubicin, may be less.
Data from the Cancer and Leukemia Group B trial 8541 (companion
8869) suggest that, with proper controls in high-volume laboratories,
many of the available methods produce comparable results.
—Ann Thor, MD
Ann Oncol 2001;(Suppl 1):S101-S107.
Molecular targets for determining HER2 status
and the tests used to detect these molecules. (mRNA = messenger
RNA, ECD = extracellular domain, ELISA = enzyme-linked immunosorbent
assay, IHC = immunohistochemistry, CISH = chromogenic in situ hybridization,
PCR = polymerase chain reaction, and FISH = fluorescence in situ
hybridization)

Adapted from Schaller et al. Ann Oncol 2001;12:(Suppl 1):S97-S100

* = calculated, ACIS™= automated
cellular imaging system, CTA= clinical trial assay (4D5 and CB11
antibodies)
Frequency of IHC scores according to level of
HER2 gene amplification.
(Pauletti G et al. J Clin Oncol 2000 ; 18:3651-3664)
 |

Level of HER-2 Gene
Amplification (HER2/neu signals per cell)
|
|
Response rates for weekly
trastuzumab and paclitaxel according to HER2 expression . (Seidman
AD et al. J Clin Oncol 2001; 19:2587-2595)
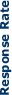 |
HER2 Status
|
|
Percent of Patients with HER2 Gene Amplification
According to Immunohistochemistry Score (IHC)
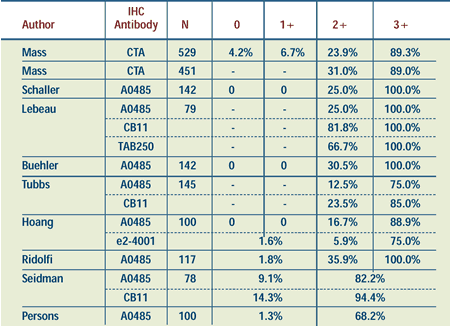
CTA = clinical trial assay (4D5 and
CB11 antibodies)
Overall response rates for trastuzumab
trials in breast cancer according to IHC score. (t=trastuzumab,
A=anthracycline, C=cyclophosphamide)

1. Cobleigh MA et al. J Clin Oncol 1999;17:2639-2648.
Abstract
2. Herceptin® [Package Insert], 2000.
3. Uber KA et al. , Pro ASCO 2001; Abstract
1949.
4. Burstein HJ et al. J Clin Oncol 2001;19:2722-2730. Abstract
View References
Back | Top of Page

|



