|
 Home:
Meeting
Highlights: Posters
Home:
Meeting
Highlights: Posters

Rationale
for Proposed NSABP Adjuvant Trial (B-35)
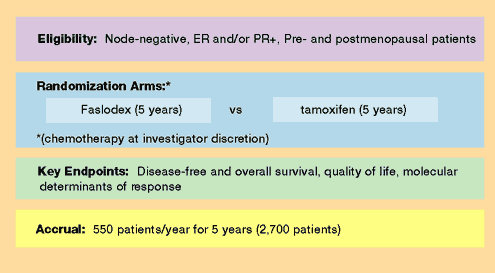
A Randomized Trial Comparing 5 Years of Faslodex® (fulvestrant)
to 5 years of Tamoxifen in Women with Hormone Receptor - Positive,
Node-negative Breast Cancer
Richard Elledge, MD
| Background:
Prior related NSABP trials |
NSABP
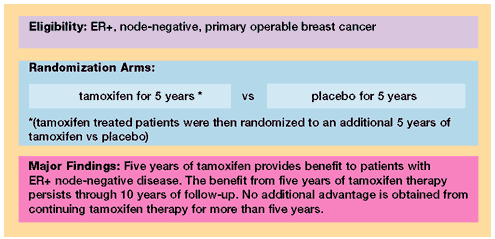
B-14: A Randomized Clinical Trial Evaluating Tamoxifen in the
Treatment of Patients with Node-Negative Breast Cancer Who Have
Estrogen Receptor-Positive Tumors
NSABP B-20:
Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive
breast cancer

Proposed
NSABP B-35 Adjuvant Trial:
(Pending the following)
A.
Demonstration of an advantage for Faslodex compared to tamoxifen
in metastatic disease trials to be reported in the fall of 2001.
B.
Demonstration of efficacy in ongoing Phase II trials involving
premenopausal patients with metastatic disease.
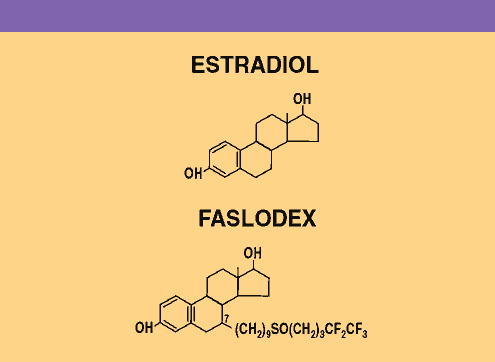
Faslodex is
a novel antiestrogen without known agonist activity. Unlike nonsteroidal
compounds, such as tamoxifen, Faslodex is a steroidal agent that
is chemically similar to estradiol. Laboratory studies have demonstrated
that Faslodex is a more potent inhibitor of tumor growth than
SERMs such as tamoxifen, and that it is capable of inhibiting
tamoxifen-resistant tumors. It is adminstered as a monthly 5 cc
intramuscular injection.
- Antagonizes
estrogen action by occupying the ER receptor and preventing
estrogen-stimulated gene activation
- Binds to
the ER with affinity similar to that of estradiol and 100 times
that of tamoxifen
- In contrast
to other SERMs, Faslodex blocks ER transactivation from both
the AF-1 and AF-2 domains
- Capable
of impairing ER dimerization and inducing degradation of the
ER, and has been termed an "estrogen receptor downregulator"
|
(a)
Mode of action of estradiol (E)
1
E binds with high affinity to the estrogen receptor (ER) to
form an E-ER complex.
2 The E-ER complex pairs with another E-ER complex (dimerizes)
and localizes in the cell nucleus. The two transcription activation
functions of ER, called AF1 and AF2, are both active.
3 The E-ER dimer binds to estrogen-sensitive genes at the
estrogen response element (ERE).
4
The dimer activates transcription of the estrogen-sensitive
gene. (AF1 and AF2 interact with coactivators to stimulate
the transcription enzyme RNA polymerase II (RNA POL II).)
(b)
Mode of action of tamoxifen (T)
1 T binds
to ER with low affinity compared with E.
|
2 The
T-ER complex dimerizes and localizes in the cell nucleus,
and AF1 (but not AF2) is active.
3 The
T-ER dimer binds to the gene DNA at the ERE.
4 Transcription
of the estrogen-sensitive gene is lessened because AF2 is
inactive, and coactivator binding is likewise attenuated.
AF1 activity results in the partial agonist activity of
T.
(c)
Mode of action of Faslodex (F)
1 F
binds to ER with affinity similar to E.
2 F
triggers rapid degradation of ER.
3 The
F-ER complex results in reduced rate of dimerization and
nuclear localization.
4 There
is reduced binding of F-ER to ERE on the gene resulting
in blocked transcription.
|
Phase III
trials comparing Faslodex versus Arimidex®
(anastrozole) in postmenopausal patients with metastatic breast
cancer
Initial results
from Trials 0020 (European) and 0021 (North American) were reported
at the 2000 San Antonio Breast Cancer Symposium. (4,5)
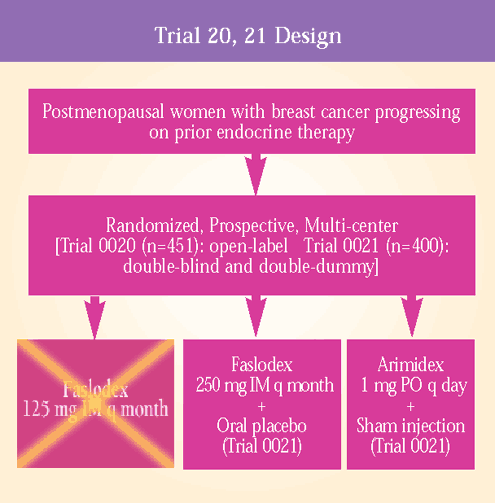
Clinical outcomes
were similar in the Faslodex and Arimidex groups. Patients receiving
Faslodex had a numerical but statistically non-significant advantage
over Arimidex in time to progression (TTP) and clinical benefit
(complete response + partial response + stable disease > 24 weeks).
Trial 0021 also demonstrated a superior median duration of response
for Faslodex (19.3 months) compared to Arimidex (10.5 months).
The incidence
of thromboembolic events was equivalent between Faslodex and Arimidex,
with a total of 15 and 17 events observed, respectively, when the
two trials were combined. Side effects were similar in both arms
including vasomotor symptoms. Essentially no significant adverse
effects were observed related to the intramuscular injection. Faslodex
is not believed to cross the blood-brain barrier and, therefore,
may not increase the rate of hot flashes. No data on bone metabolism,
fractures or other secondary effects is yet available.
Safety in
Premenopausal Women
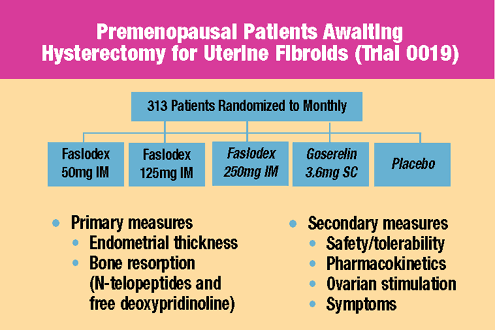
Trial
0019 evaluated
three doses of Faslodex versus goserelin and placebo in 313 premenopausal
women with uterine fibroids awaiting hysterectomy. Drug or placebo
was given for three months. Overall, this study demonstrated that
Faslodex was safe and well-tolerated in premenopausal women. Key
findings included:
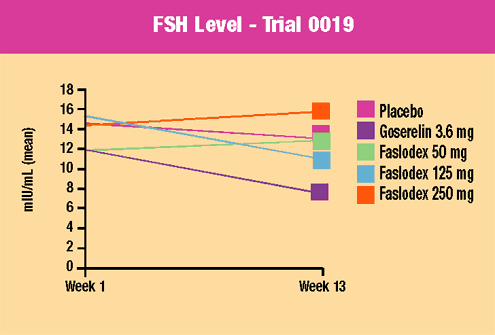
- No evidence
of menopausal symptoms
- No evidence
of ovarian hyperstimulation
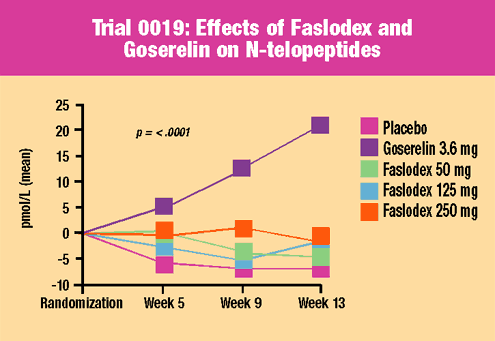
- Lower excretion
of surrogate markers of bone catabolism compared to goserelin
and similar to placebo
- No difference
in safety endpoints at three months
- Fisher B,
et al. Tamoxifen and Chemotherapy for Lymph Node-Negative, Estrogen
Receptor-Positive Breast Cancer. Journal of the National Cancer
Institute 89.22:1673-1682, 1997.
- Fisher B.
Highlights from Recent National Surgical Adjuvant Breast and Bowel
Project Studies in the Treatment and Prevention of Breast Cancer.
CA - A Cancer Journal for Clinicians 49(3):159-177, May-June 1999.
- Howell A,
et al. Comparison of Efficacy and Tolerability of Fulvestrant
(Faslodex) with Anastorozle (Arimidex) in Post-Menopausal Women
with Advanced Breast Cancer. Breast Cancer Research and Treatment
64(1):27, November 2000.
- Osborne CK,
et al. A Double-Blind Randomized Trial Comparing the Efficacy
and Tolerability of Faslodex (Fulvestrant) with Arimidex (Anastrozole)
in Post-Menopausal Women with Advanced Breast Cancer. Breast Cancer
Research and Treatment 64(1):27, November 2000.
- Robertson
J, et al. The Pharmacokinetics of Single Dose Faslodex® (ICI
182,780) in Postmenopausal Primary Breast Cancer - Relationship
with Estrogen Receptor (ER) Down Regulation. Program/Proceedings
of the American Society of Clinical Oncology 19:94a (Abstract
362). May 2000.
Top
of Page
|



