 Home:
Meeting
Highlights: Posters
Home:
Meeting
Highlights: Posters

Post-Radiation
Angio Sarcoma of the Breast
Gal-Combos EC, Esserman LE, Godinez J, Recine MA and Poppiti RJ,
Jr.
The first reported
case of angiosarcoma (AS) of the breast was in 1887 by G. B. Schmidt.
AS represents 0.04% of all malignant tumors of the breast, and 3-9%
of sarcomas of the breast. AS is a well recognized but rare complication
of radiation therapy. The first case of mammary AS following lumpectomy,
axillary lymph node dissection and irradiation was reported by Bodey
in 1987. Less than 30 cases were reported from then, mainly sporadic
case reports. Initial underdiagnosis from both the radiologic and
pathologic perspectives, is not uncommon as is illustrated by the
following examples. With the widespread use of conservative surgery
and radiation therapy increase in the incidence of postradiation
AS is expected.
We reviewed
the available clinical histories and imaging and pathologic findings
of five cases of histopathologically proven breast angiosarcomas
diagnosed from 1994 to 1999 in our institution. Four of them developed
in the irradiated field after radiotherapy for breast cancer and
one patient had primary AS of the breast.
Patients
data:
The five patients,
all women, were identified from the search of pathology records.
The age at diagnosis was 42-85 years (mean: 63 years).
Interval from
irradiation until diagnosis of AS:
The range was
3 11/12 years to 8 2/12 years (mean 6 7/12 years).
Clinical
presentation:
The clinical
examination showed palpable mass or masses in all the cases with
purple discoloration of the skin at the surgical scar in three cases.
Two lesions were painful but soft on physical examination. Two cases
were clinically misinterpreted as local recurrence of the original
breast carcinoma and one case was followed almost for a year as
"multiple hematomas, ecchymoses of the breast".

Imaging findings:
Mammography
revealed well-defined subcutaneous mass in two cases. In two cases
the mammogram revealed only diffuse thickening of the skin. Ultrasound
(in one case) showed an irregular hypo-echoic lesion with a thick-walled
echogenic rim.
Gross pathologic
findings:
Gross examination
showed pink, red or purple, hemorrhagic, ill-defined lesions in
the subcutaneous breast tissue. One lesion was more solid and white
and the vascular nature was seen only microscopically.
Histopathologic
findings:
Two features
are characteristic: anastomosing vascular channels within the breast
parenchyma and prominent, hyperchromatic endothelial cells.
For the
classification, three grades (or types) were described by Rosen
et. al., based on microscopic features:
Grade I: Low-grade
tumor entirely consists of well-formed anastomosing vascular channels
that invade the breast parenchyma. The cells tend to be flat and
there is little or no papillary endothelial proliferation. Few mitotic
figures are seen.
|
Figure
1A
|
Figure
1B
|
 |
 |
|
Figure
2A
|
Figure
2B
|
 |
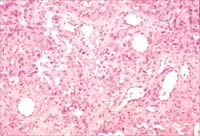 |
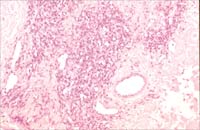
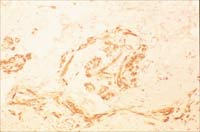
Fig1A.
Vascular proliferation in low grade AS (Case#2) (H and E x200).
Fig 1B. CD 34 (an endothelial marker) was positive, showing that
the lesion is vascular (x200)
Grade II: Intermediate
grade tumor consists of largely of a Grade I proliferation. Scattered
in the lesion are foci of more solid vascular growth that have papillary
endothelial and/or solid components.
Grade III: High-grade
tumor is characterized by prominent areas of spindle cell sarcoma
or papillary and solid endothelial patterns. Focal necrosis, hemorrhage
and a numerous mitotic figures occur.
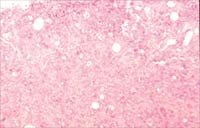
Fig
2A. High grade AS (Case#4) with spindle cells and endothelial proliferation
(H and E x100).
Fig
2B. Highly atypical cells with mitotic figures (H and E x200).
Cytologic
and histopathologic misdiagnosis:
Rainwater et
al reported a recent decrease in underdiagnosis from 64% to 33%.
Chen et al stressed that the significant percentage of misdiagnoses
of AS of the breast continues with an incidence of as high as 39%.
In this study
FNA was performed in two cases. In the first case ultrasound guided
fine needle aspiration was positive for "carcinoma" cells.
In the other case FNA was negative for malignant cells. The detected
clusters of ductal and spindle cells suggested fibroadenoma. Due
to this deceptive benign appearance the diagnosis was postponed
by 4 months.
Histopathology
of the ultrasound guided core biopsy misdiagnosed the AS as infiltrating
duct cell carcinoma in one occasion (Case#1). In contrast to this,
the mastectomy showed a high-grade angiosarcoma.
Figure
3. (Case#1) 58-year-old woman who presented with pain in the left
breast. Prior biopsy of the ipsilateral breast, performed six years
ago, revealed a focus of infiltrating carcinoma, duct cell type,
with extensive intraductal carcinoma extending to the surgical margin.
The patient was treated with lumpectomy and 35 doses of 1.8 Gy whole
breast irradiation therapy, the total of 63Gy.
The
current physical examination showed a purple discoloration of the
skin at the surgical scar and a palpable, mobile mass.
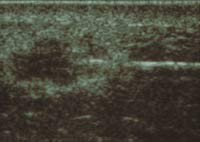
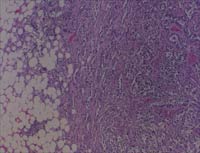
Fig
3A. and B. Mammography and ultrasound studies revealed an irregular
subcutaneous mass and a 1.6 x 1.0cm hypo-echoic lesion with a thick-walled
echogenic rim respectively. Subsequent ultrasound guided fine needle
aspiration and ultrasound-core biopsies were diagnosed as positive
for carcinoma cells and infiltrating duct cell carcinoma respectively.
A mastectomy was performed.
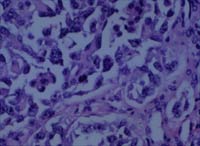
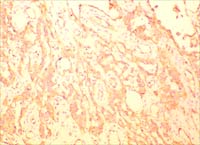
Fig 3C. and D. In contrast to the previous pathologic findings,
the mastectomy showed a 1.4 cm high-grade angiosarcoma with prominent
epithelioid features and a high mitotic index (H and E x100 and
x400).
Fig
3E. The vascular differentiation of the tumor was confirmed with
positive immunohistochemical staining for CD31 (Figure x200), CD34,
Factor VIII and Ulex Europeaus and negative staining for cytokeratin
markers. Retrospective immunohistochemical staining of the previously
mentioned ultrasound-core biopsies showed a similar staining pattern.
Mastectomy provided
the diagnosis in the remaining cases.
Size of the
AS:
The size of
the tumor varied at the time of initial presentation from 1.4 cm
to diffuse involvement of the breast, including a huge mass, larger
than 22 cm.
Treatment:
Mastectomy was
performed in all the cases and the patients received adjuvant chemotherapy.
In one case, local radiation therapy was added.
Outcome:
AS of the breast
is a rare disease and often has a fatal outcome. Two patients died
within 3 years of diagnosis. One of the survivors had subcutaneous
recurrence at a distant site (forehead) after 40 months. The other
survivor has no evident disease at 17 months.
Several possible
explanations have been proposed for the etiology of radiation-associated
AS. Malignant change can be induced in a primary benign lesion,
such as hemangioma. Alternatively, a new tumor may develop regardless
of the primary tumor because of irradiation. The chronic lymphedema
caused by extensive axillary node dissection also may be an important
risk factor for the onset of AS (Stewart-Treves syndrome).
In AS of the
breast there are no definitive diagnostic and treatment clues. Although
the diagnostic pitfalls have been mentioned in several previous
reports, the problem of misdiagnosis still occurs in the recently
reported cases. AS should be included in the differential diagnosis
in cases after irradiation of the breast especially when a vascular
lesion is associated with any breast mass.
|
Figure
3A
|
 |
|
Figure
3D
|
 |
|
|
Figure
3B
|
 |
| |
|
Figure
3C
|
 |
 |
|
Figure
3E
|
 |
|
1. Polgar C,
Orosz Z, Szerdahelyi A, Fodor J, Major T, Magori A, Czeyda-Pommersheim
F, Vamosi Nagy I, Szakolczai I, Fejos Z, Nemeth G Postirradiation
Angiosarcoma of the Chest Wall and Breast: Issues of Radiogenic
Origin, Diagnosis and Treatment in Two Cases. Oncology 2001 Dec;60(1):31-34
2. Kagawa Y,
Saeki T, Takiyama W, Takashima S, Mandai K Angiosarcoma of the Breast:
Report of Case and Autopsy Findings. Breast Cancer 1997 Mar 25;
4(1):33-37
3. Yang WT,
Muttarak M, Ho LW Nonmammary malignancies of the breast: ultra sound,
CT, and MRI. Semin Ultrasound CT MR 2000 Oct;21(5):375-94
4. Majeski J,
Austin RM, Fitzgerald RH Cutaneous angiosarcoma in an irradiated
breast after breast conservation therapy for cancer: association
with chronic breast lymphedema. J Surg Oncol 2000 Jul;74(3):208-12;
discussion 212-3
5. Rosen PP,
Kimmel M, Ernsberger D Mammary angiosarcoma. The prognostic significance
of tumor differentiation. Cancer 1988 Nov 15;62(10):2145-51
6. Donnell RM,
Rosen PP, Lieberman PH, Kaufman RJ, Kay S, Braun DW Jr, Kinne DW
Angiosarcoma and other vascular tumors of the breast. Am J Surg
Pathol 1981 Oct;5(7):629-42
7. Strobbe LJ,
Peterse HL, van Tinteren H, Wijnmaalen A, Rutgers EJ Angiosarcoma
of the breast after conservation therapy for invasive cancer, the
incidence and outcome. An unforseen sequela. Breast Cancer Res Treat
1998 Jan;47(2):101-9
8. Rainwater
LM, Martin JK Jr, Gaffey TA, van Heerden JA Angiosarcoma of the
breast. Arch Surg 1986 Jun;121(6):669-72
9. Chen KT,
Kirkegaard DD, Bocian JJ Angiosarcoma of the breast. Cancer 1980
Jul 15;46(2):368-71
Top
of Page
|



