|
 Home:
Meeting
Highlights: Posters
Home:
Meeting
Highlights: Posters

Current NSABP Breast Cancer Trials
Current
NSABP Breast Cancer Trials
Richard Kaderman, Ph.D.
History:
The National
Surgical Adjuvant Breast and Bowel Project (NSABP) is a cooperative
group that was formally established in 1971 to conduct clinical
trials in breast and colorectal cancer research; however, members
have been involved in collaborative research since 1958.
Over 50,000
women and men have been enrolled in NSABP clinical treatment trials.
In the Breast Cancer Prevention Trial, over 100,000 women were screened
and 13,388 were enrolled.
|
Organization:
- NSABP
Operations Center: Norman Wolmark, MD, Principal Investigator
East Commons Professional Bldg. Four Allegheny Center -
5th Floor Pittsburgh, PA 15212-5234 Phone: (412) 330-4600
Fax: (412) 330-4660
- NSABP
Biostatistical Center: Dr. H. Samuel Wieand, Director University
of Pittsburgh One Sterling Plaza 201 North Craig Street,
Suite 500 Pittsburgh, PA 15213 Phone: (412) 624-2666 Fax:
(412) 624-1082
|

Bernard
Fisher, MD, Scientific Director, NSABP
|

NSABP group
photo, circa early 1980's
Membership:
Members include
300 medical centers in the United States, Canada and Australia,
and over 6000 physicians, nurses and other medical professionals
in the NSABP member institutions and their satellites. Institutions
include major medical centers, university hospitals, large oncology
practice groups and health maintenance organizations.
Funding:
The National
Cancer Institute (NCI) is the primary source of funding for NSABP
clinical trials and the Operations and Biostatistical Centers. Other
sources fund ancillary studies, training and educational programs.
| Impact
of Select NSABP Clinical Trials on Breast Cancer Management
|
Emergence
of breast-conserving surgery as standard of care. These findings
contributed to the 1990 NIH consensus conference recommendation
that lumpectomy and breast irradiation be the preferred procedure
for women with primary breast cancer.
Fisher
B, Anderson S, Redmond C, et al. Reanalysis and Results after 12
Years of Follow-up in a Randomized Clinical Trial Comparing Total
Mastectomy with Lumpectomy with or without Irradiation in the Treatment
of Breast Cancer. New England Journal of Medicine 333:22:1456-1461,
1995.
First
demonstration that systemic therapy could alter the natural history
of primary breast cancer.
Fisher
B, Redmond C, Brown A, et al. Treatment of Primary Breast Cancer
with Chemotherapy and Tamoxifen. New England Journal of Medicine
305:1-6, 1981.
The
benefits of adjuvant tamoxifen for women with invasive and noninvasive
breast cancer.
Fisher B,
Costantino J, Redmond C, et al. A Randomized Clinical Trial Evaluating
Tamoxifen in the Treatment of Patients with Node-Negative Breast
Cancer Who Have Estrogen Receptor-Positive Tumors. New England
Journal of Medicine 320:479-484, 1989.
Fisher B,
Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal
breast cancer: National Surgical Adjuvant Breast and Bowel Project
B-24 randomised controlled trial. Lancet 353(9169):1993-2000,
June 12, 1999.
Fisher B,
Dignam J, Bryant J, et al. Five Versus More Than Five Years of
Tamoxifen Therapy for Breast Cancer Patients with Negative Lymph
Nodes and Estrogen Receptor-Positive Tumors. Journal of the National
Cancer Institute 88:1529-42, 1996.
First
demonstration of improved disease-free and overall survival due
to adjuvant therapy in node-negative breast cancer patients, including
women with very small tumors.
Fisher
B, Dignam J, Tan-Chiu E, et al. Prognosis and Treatment of Patients
With Breast Tumors of One Centimeter or Less and Negative Axillary
Lymph Nodes Journal of the National Cancer Institute 93: 112-120,
2001
Fisher
B, Dignam J, Mamounas EP, et al. Sequential Methotrexate 5-Fluorouracil
(M_F) for the Treatment of Node-Negative Breast Cancer Patients
with Estrogen Receptor-Negative Tumors: Eight-Year Results from
NSABP B-13 and First Report of Findings from NSABP B-19 Comparing
M_F with Conventional CMF. Journal of Clinical Oncology 14:7:1982-1992,
1996.
Fisher
B, Dignam J, Wolmark N, et al. Tamoxifen and Chemotherapy for Lymph
Node-Negative, Estrogen Receptor-Positive Breast Cancer. Journal
of the National Cancer Institute 89.22:1673-1682, 1997.
Fisher
B, Dignam J, Wolmark N, et al. Tamoxifen and Chemotherapy for Lymph
Node-Negative, Estrogen Receptor-Positive Breast Cancer. Journal
of the National Cancer Institute 89.22:1673-1682, 1997.
Combination
chemohormonal therapies improve disease-free and overall survival
in node-negative, receptor-positive breast cancer patients.
Fisher B,
Dignam J, Wolmark N, et al. Tamoxifen and Chemotherapy for Lymph
Node-Negative, Estrogen Receptor-Positive Breast Cancer. Journal
of the National Cancer Institute 89.22:1673-1682, 1997.
Preoperative
chemotherapy results in significant downstaging in axillary nodal
status (80% response rate) and an increase in breast-conserving
treatment, with improved disease-free and overall survival in patients
who achieved a complete pathological response.
Fisher
B, Bryant J, Wolmark N, et al. Effect of Preoperative Chemotherapy
on the Outcome of Women with Operable Breast Cancer. Journal of
Clinical Oncology 16(8):2672-2685, 1998.
Tamoxifen
reduces the risk of developing breast cancer by 50% or more in high-risk
women, prompting the FDA to grant approval for the drug as the first
chemopreventive agent for breast cancer.
Fisher
B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention
of breast cancer: report of the National Surgical Adjuvant Breast
and Bowel Project P-1 Study. Journal of the National Cancer Institute
90:18:1371-1388, 1998.
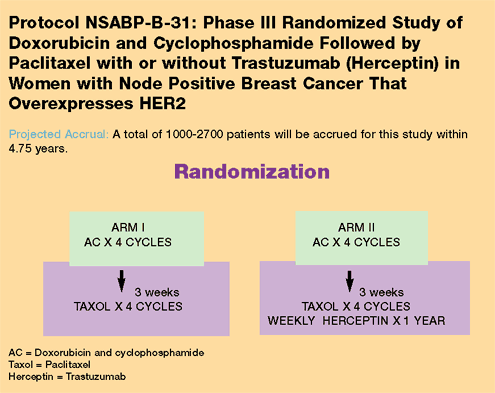
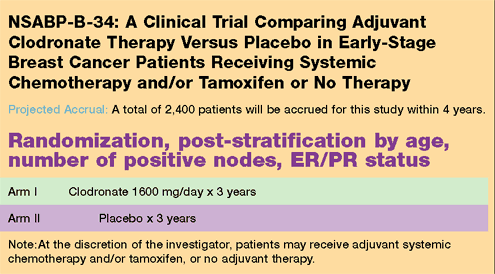
| Current
NSABP Breast Cancer Trials |
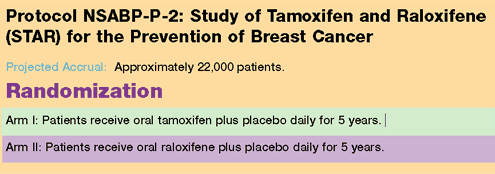
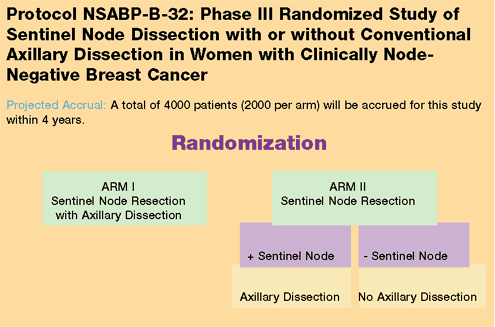
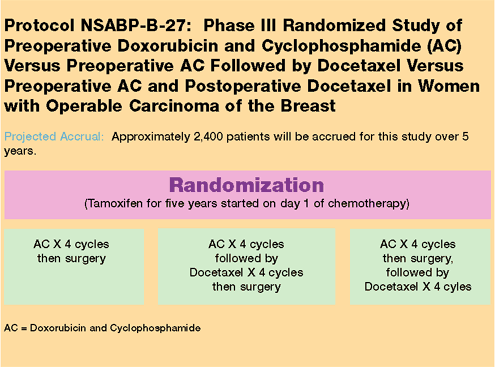
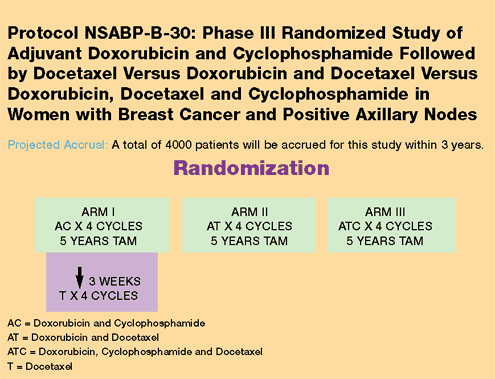
For additional
information visit the NSABP website at http://www.nsabp.pitt.edu/
Top
of Page
|



