|

 DR PAPISH: This patient is the 45-
year-old premenopausal wife of a physician
in our community who presented
with a left breast abnormality that she
felt on examination. She underwent
mammography, which was negative,
but an ultrasound revealed a seven-millimeter
nodule at approximately five
o’clock. She ultimately underwent an
excisional biopsy for a 0.7-centimeter,
poorly differentiated, invasive ductal
carcinoma that contained approximately
25 percent high-grade DCIS. DR PAPISH: This patient is the 45-
year-old premenopausal wife of a physician
in our community who presented
with a left breast abnormality that she
felt on examination. She underwent
mammography, which was negative,
but an ultrasound revealed a seven-millimeter
nodule at approximately five
o’clock. She ultimately underwent an
excisional biopsy for a 0.7-centimeter,
poorly differentiated, invasive ductal
carcinoma that contained approximately
25 percent high-grade DCIS.
The tumor was strongly ER-positive
(90 percent on IHC), PR-positive (80
percent), and HER2-positive (amplified
by FISH). She subsequently underwent
bilateral breast MRIs, which were
negative. Her family history is negative
for breast cancer and ovarian cancer, but
she is of Ashkenazi heritage and underwent
both BRCA1 and BRCA2 testing
for the three Ashkenazi genes.
 DR LOVE: John, if this woman turned
out to be BRCA1- or BRCA2-positive,
would you approach her local breast
cancer therapy any differently? DR LOVE: John, if this woman turned
out to be BRCA1- or BRCA2-positive,
would you approach her local breast
cancer therapy any differently?
 DR MACKEY: When we see a patient
who is BRCA1-positive, we often have
the discussion that they’re at quite high
risk of local recurrence or a second
primary cancer. Even though this is
a small tumor, the woman should be
given the discussion regarding prophylactic
mastectomy, as well as perhaps
a completion mastectomy on the
affected side. DR MACKEY: When we see a patient
who is BRCA1-positive, we often have
the discussion that they’re at quite high
risk of local recurrence or a second
primary cancer. Even though this is
a small tumor, the woman should be
given the discussion regarding prophylactic
mastectomy, as well as perhaps
a completion mastectomy on the
affected side.
 DR PAPISH: She then underwent a
re-excision and sentinel node biopsy.
The re-excision revealed a residual two-millimeter
area of just high-grade DCIS
with no other invasive tumor. Three
sentinel lymph nodes were negative by
IHC and H&E. DR PAPISH: She then underwent a
re-excision and sentinel node biopsy.
The re-excision revealed a residual two-millimeter
area of just high-grade DCIS
with no other invasive tumor. Three
sentinel lymph nodes were negative by
IHC and H&E.
 DR LOVE: What was her attitude? Was
she more of the proactive, “I want to do
everything possible,” or was she more
concerned about toxicity? DR LOVE: What was her attitude? Was
she more of the proactive, “I want to do
everything possible,” or was she more
concerned about toxicity?
 DR PAPISH: There was a clear-cut
difference between the patient and her
husband. She is a long-distance runner.
She was very worried about anything
that might impact on her cardiovascular
health. She truly did not want to
receive any aggressive therapy if it were
not warranted. However, she’s bright
enough to understand that if it were
needed, she would accept it. DR PAPISH: There was a clear-cut
difference between the patient and her
husband. She is a long-distance runner.
She was very worried about anything
that might impact on her cardiovascular
health. She truly did not want to
receive any aggressive therapy if it were
not warranted. However, she’s bright
enough to understand that if it were
needed, she would accept it.
 DR LOVE: Aman, would you bring up
the use of the Oncotype DX assay in
this woman? DR LOVE: Aman, would you bring up
the use of the Oncotype DX assay in
this woman?
 DR BUZDAR: The tumor is relatively
small, and the risk in this woman of
this breast cancer causing a problem is
relatively small. But if she has one breast
cancer, she has at least a 0.6 percent risk
of developing contralateral breast cancer
on a yearly basis, and she’s young. She
may have another 30 or 40 years of
lifespan left. So that also has to be taken
into account. DR BUZDAR: The tumor is relatively
small, and the risk in this woman of
this breast cancer causing a problem is
relatively small. But if she has one breast
cancer, she has at least a 0.6 percent risk
of developing contralateral breast cancer
on a yearly basis, and she’s young. She
may have another 30 or 40 years of
lifespan left. So that also has to be taken
into account.
 DR LOVE: So how did you approach
this patient? DR LOVE: So how did you approach
this patient?
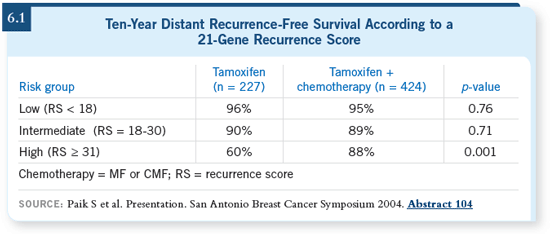
 DR PAPISH: We discussed the
Oncotype DX assay. My bias was that
with a poorly differentiated tumor and
borderline but HER2-positive amplification,
we would probably see a high or
a high-intermediate recurrence score.
The recurrence score was 16 (6.1). DR PAPISH: We discussed the
Oncotype DX assay. My bias was that
with a poorly differentiated tumor and
borderline but HER2-positive amplification,
we would probably see a high or
a high-intermediate recurrence score.
The recurrence score was 16 (6.1).
 DR LOVE: Her recurrence score was 16,
which is low risk. What did you decide
to do? DR LOVE: Her recurrence score was 16,
which is low risk. What did you decide
to do?
 DR PAPISH: She was started on
tamoxifen and breast irradiation. We
had previously discussed that if the
recurrence score from the Oncotype DX
assay was low, we would use hormonal
therapy. DR PAPISH: She was started on
tamoxifen and breast irradiation. We
had previously discussed that if the
recurrence score from the Oncotype DX
assay was low, we would use hormonal
therapy.
I explained that I had a bias about
possibly using an aromatase inhibitor
and ovarian ablation but did not feel
strongly that it needed to be done at
the moment. She’s perimenopausal, so
she clearly would not be a candidate for
an aromatase inhibitor alone at present.
They have an appointment next week,
and her husband still thinks he may
want her to have chemotherapy. But I
think the patient is very comfortable
with hormonal therapy.
 DR LOVE: If you’re thinking about
chemotherapy, potentially you also
might be talking about trastuzumab. DR LOVE: If you’re thinking about
chemotherapy, potentially you also
might be talking about trastuzumab.
 DR PAPISH: The tumor is FISH-positive,
but I don’t know whether the
Oncotype DX assay trumps the HER2
positivity in the sense that we have a
relatively low recurrence score. I would
presume that if she had an increase in
genes coding for HER2 positivity, she
would have had a higher recurrence
score and certainly a more proliferative
tumor. DR PAPISH: The tumor is FISH-positive,
but I don’t know whether the
Oncotype DX assay trumps the HER2
positivity in the sense that we have a
relatively low recurrence score. I would
presume that if she had an increase in
genes coding for HER2 positivity, she
would have had a higher recurrence
score and certainly a more proliferative
tumor.
 DR LOVE: John, I’m curious. Is it
possible that the HER2 positivity is
from the DCIS? DR LOVE: John, I’m curious. Is it
possible that the HER2 positivity is
from the DCIS?
 DR MACKEY: There’s generally a high
degree of concordance between the
HER2 status of DCIS and invasive
disease. In general, the two travel
together. However, when one does
FISH, the person who’s reading it
should be trained enough to score only
the invasive nuclei, as opposed to the in
situ component. DR MACKEY: There’s generally a high
degree of concordance between the
HER2 status of DCIS and invasive
disease. In general, the two travel
together. However, when one does
FISH, the person who’s reading it
should be trained enough to score only
the invasive nuclei, as opposed to the in
situ component.
 DR LOVE: Andy, what would you say
to this woman? DR LOVE: Andy, what would you say
to this woman?
 DR SEIDMAN: I think we need to be
cognizant of what the real benefit is
and lead with the data rather than the
emotional response. I would absolutely
give this woman tamoxifen and talk
to her about participation in a trial of
ovarian function suppression. DR SEIDMAN: I think we need to be
cognizant of what the real benefit is
and lead with the data rather than the
emotional response. I would absolutely
give this woman tamoxifen and talk
to her about participation in a trial of
ovarian function suppression.
 DR LOVE: You’re going to knock down
the relapse rate a little bit with trastuzumab.
Of course, following an anthracycline
base, we’re looking at probably a three or four percent risk of cardiomyopathy.
A young woman who’s a jogger
probably has normal cardiac function. If
the husband brings that up, Andy, how
would you respond? DR LOVE: You’re going to knock down
the relapse rate a little bit with trastuzumab.
Of course, following an anthracycline
base, we’re looking at probably a three or four percent risk of cardiomyopathy.
A young woman who’s a jogger
probably has normal cardiac function. If
the husband brings that up, Andy, how
would you respond?
 DR SEIDMAN: I think the only
meaningful data we have regarding
trastuzumab’s role in patients with
node-negative breast cancer come from
the HERA trial, in which there were
1,100 such patients randomly assigned
to trastuzumab or not (Piccart-Gebhart
2005). They had to have T1C or greater
tumors; this woman clearly doesn’t fit
that category. The incidence of cardiac
events is probably very close to the
potential benefit she would receive. It
would be easy to defend not giving her
trastuzumab. DR SEIDMAN: I think the only
meaningful data we have regarding
trastuzumab’s role in patients with
node-negative breast cancer come from
the HERA trial, in which there were
1,100 such patients randomly assigned
to trastuzumab or not (Piccart-Gebhart
2005). They had to have T1C or greater
tumors; this woman clearly doesn’t fit
that category. The incidence of cardiac
events is probably very close to the
potential benefit she would receive. It
would be easy to defend not giving her
trastuzumab.
 DR LOVE: Aman, how would you
approach the issue of chemotherapy and
maybe even trastuzumab in this patient? DR LOVE: Aman, how would you
approach the issue of chemotherapy and
maybe even trastuzumab in this patient?
 DR BUZDAR: I think the trastuzumab
part is a little tricky, because she has a
small tumor where 20 or 25 percent of
the tumor is DCIS. I agree with John,
that pathologists have to carefully look
at and make sure that this overamplification
of HER2 is indeed in the
invasive component. Sometimes our
pathologists at MD Anderson will say,
“Yes, there is overamplification, but it
is only confined in the DCIS area.” We
should not be misguided and overtreat
the patient. DR BUZDAR: I think the trastuzumab
part is a little tricky, because she has a
small tumor where 20 or 25 percent of
the tumor is DCIS. I agree with John,
that pathologists have to carefully look
at and make sure that this overamplification
of HER2 is indeed in the
invasive component. Sometimes our
pathologists at MD Anderson will say,
“Yes, there is overamplification, but it
is only confined in the DCIS area.” We
should not be misguided and overtreat
the patient.
What is interesting and intriguing
about this patient is that she is young
— she’s 45 — so she has a fairly long
lifespan. But the tumor is very small
and is strongly ER/PR-positive. The
addition of chemotherapy in patients
with strongly ER/PR-positive disease
is marginal at best; most of the benefit
in this patient would be from endocrine
intervention.
 DR RAVDIN: This case represents where
molecular characterization comes into
play in terms of treatment sensitivity.
Even though this patient had a low-risk
Oncotype DX score — seven percent
rate of distant recurrence — that range
includes patients with as low as two
percent or as high as 10 percent risk.
And a 10 percent risk of distant recurrence
is — if you’re the one facing it
— a substantial risk. DR RAVDIN: This case represents where
molecular characterization comes into
play in terms of treatment sensitivity.
Even though this patient had a low-risk
Oncotype DX score — seven percent
rate of distant recurrence — that range
includes patients with as low as two
percent or as high as 10 percent risk.
And a 10 percent risk of distant recurrence
is — if you’re the one facing it
— a substantial risk.
The Overview says chemotherapy
adds to such patients about a 30
percent relative benefit. Now, a lot of
that benefit — at least half of it — is
probably an endocrine ablation type of
benefit. But nonetheless, it is a benefit.
So you could argue rationally that such
a patient should be treated if you believe
that she would get 30 percent of 10
percent, or a three percent net benefit.
However, a proportional benefit for this
woman is likely to be less than that 30
percent benefit. In the future, I think if
you look at it as a three percent possible
benefit, that would be attractive to a
number of people. So I don’t think you
can discount the idea that such a patient
would be treated even if all you know is
the prognostic information.
 DR LOVE: One final question for
Aman. Does the fact that this patient
has FISH-positive disease make you
concerned about using just tamoxifen
as opposed to an LHRH agonist or
an LHRH agonist plus an aromatase
inhibitor? DR LOVE: One final question for
Aman. Does the fact that this patient
has FISH-positive disease make you
concerned about using just tamoxifen
as opposed to an LHRH agonist or
an LHRH agonist plus an aromatase
inhibitor?
 DR BUZDAR: There are small but
definite data emerging that suggest
that patients who have HER2-positive
disease may have better disease-free
survival when treated with an aromatase
inhibitor — both in the metastatic
and the adjuvant settings, or even in
the neoadjuvant setting (Dowsett 2005,
Smith 2005). DR BUZDAR: There are small but
definite data emerging that suggest
that patients who have HER2-positive
disease may have better disease-free
survival when treated with an aromatase
inhibitor — both in the metastatic
and the adjuvant settings, or even in
the neoadjuvant setting (Dowsett 2005,
Smith 2005).
Select publications
|

