|
You are here: Home: BCU 9|2004: Grand Rounds: Adam M Brufsky, MD, PhD

* Originally presented at a Breast Cancer Update Working Group meeting, Naples, October 2, 2004. For the audio portion of this presentation and the PowerPoint slides, please see accompanying CDs.
| 4.3 |
|
| |
|

SLIDE 4.2 I’m not sure there is an optimal sequence of hormonal therapy in metastatic disease. From recently updated SEER data, we know that approximately 23 percent of women with metastatic breast cancer survive at least five years. Many of them have slowly progressing disease that can be adequately controlled with hormones. I think the art of this is to decide which hormones to use and how.
SLIDE 4.3 It’s gratifying that systemic therapy is doing better. The British Columbia group evaluated their database of patients with metastatic breast cancer and found the median survival actually improved by about 28 percent over a 10-year period. This median survival benefit continues to improve in metastatic disease. The supportive care we’re providing is better, and we’re diagnosing metastatic disease earlier in its course. On the other hand, the addition of novel and more improved systemic therapies has had something to do with this improvement in survival, particularly the introduction of nonsteroidal aromatase inhibitors in the mid 1990s.
|
| |
|
|
| 4.4 |
|
| |
|
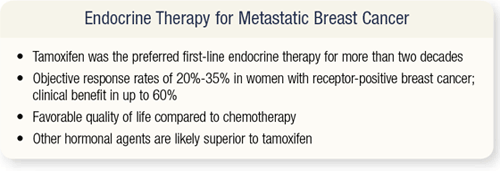
SLIDE 4.4 Tamoxifen was the preferred agent for the treatment of metastatic breast cancer for more than two decades. Because the vast majority of women with metastatic breast cancer have bone and softtissue disease, it’s often difficult to measure objective response to hormonal therapies. Presently, the objective response of these women to endocrine therapy is about 35 percent. A study of an aromatase inhibitor versus megesterol demonstrated that a clinical benefit that is a partial response, complete response or stable disease greater than six months is a good surrogate endpoint for survival. A lot of us say, “Gosh, if a woman’s cancer didn’t get better in six months, what are we really doing?”
A trial of anastrozole versus megesterol as second-line therapy for metastatic disease showed that the two-year survival rate was 85 percent in women who had stable disease for six months or greater — the same two-year survival rate seen in women who had complete or partial response. That’s good to know when we try to evaluate the literature or counsel patients. I have patients with metastatic disease who come to me very upset and say, “My cancer didn’t get any better. Let’s switch therapy.” I tell them, “On the other hand, you have had stable disease for more than six months, so your chance of survival at two years is the same as it would be if all your cancer went away.” It’s hard to convince them of that.
|
| |
|
|
| 4.5 |
|
| |
|

SLIDE 4.5 What about using aromatase inhibitors? Are they superior to tamoxifen?
|
| |
|
|
| 4.6 |
|
| |
|
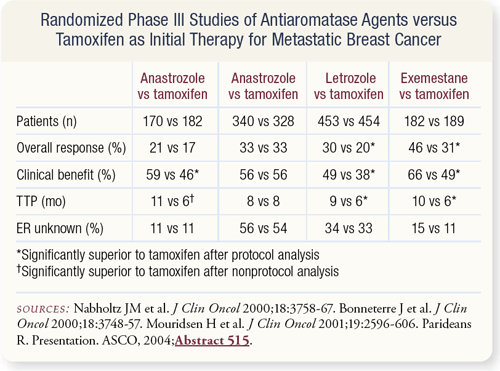
SLIDE 4.6 The available data suggest that aromatase inhibitors are better than tamoxifen as first-line therapy for metastatic disease.
|
| |
|
|
| 4.7 |
|
| |
|

SLIDE 4.7 We know that aromatase inhibitors are likely superior to tamoxifen as first-line therapy. They’re clearly superior to megesterol following tamoxifen failure. We know that patients can respond to a steroidal aromatase inhibitor after failing a nonsteroidal aromatase inhibitor, but what about the use of other agents such as the estrogen receptor downregulator fulvestrant.
|
| |
|
|
| 4.9 |
|
| |
|

SLIDE 4.9 When estrogen binds to the estrogen receptor in the cytoplasm, the receptors dimerize and then go into the nucleus. In the nucleus, cofactors interact with the “business” end of the molecule, called AF1, to activate gene transcription. These cofactors are different depending on the tissue type. Miles Brown, whom I used to work for as a post-doc, elaborated on this a lot in the 1990s, and it is very interesting.
|
| |
|
|
| 4.10 |
|
| |
|
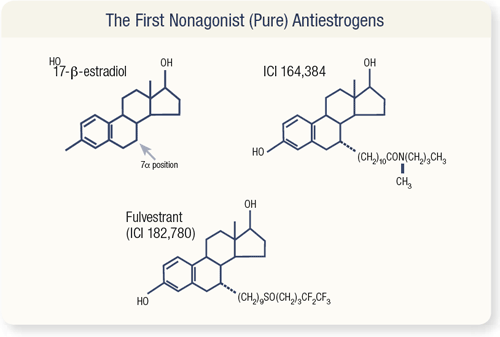
SLIDE 4.10 To produce fulvestrant, estradiol is modified with a long side group at the seven alpha position.
|
| |
|
|
| 4.11 |
|
| |
|
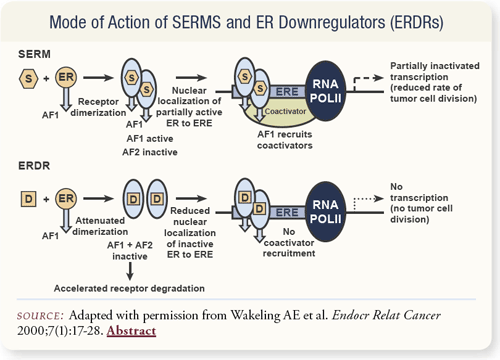
SLIDE 4.11 Estrogen receptor downregulators block dimerization in the cytoplasm and completely degrade the receptors. This is unlike SERMs, such as raloxifene, which allow the receptors to dimerize, but block the activation of cofactors in the nucleus, resulting in only a partial disruption of gene expression and cell growth.
|
| |
|
|
| 4.12 |
|
| |
|

SLIDE 4.12 In animal experiments, when a breast cancer cell line is exposed to tamoxifen, immunohistochemical analysis shows the receptors are still present. After exposure to fulvestrant, all the receptors disappear in the nucleus. This seems to be a purer way of affecting estrogen receptor function.
Cells often become resistant to toremifene and tamoxifen over time and tend to grow when given these drugs. If given fulvestrant, they don’t grow at all. When given fulvestrant and the supposed stimulus of tamoxifen/toremifene, the result is partial suppression. Clearly, some cross-resistance occurs, and perhaps something could be done to the existing receptor in the cell to overcome that resistance. One hypothesis is that the receptor becomes mutated in the cell. Fulvestrant can partially overcome this resistance, which suggests it can be used after tamoxifen failure.
|
| |
|
|
| 4.14 |
|
| |
|

SLIDES 4.13 - 4.14 Data from head-to-head Phase III trials of fulvestrant versus anastrozole following tamoxifen failure demonstrate overall response rates and duration of response to be similar. Such results suggest that in postmenopausal women with metastatic breast cancer following tamoxifen failure, aromatase inhibitors and fulvestrant are essentially equal.
|
| |
|
|
| 4.15 |
|
| |
|
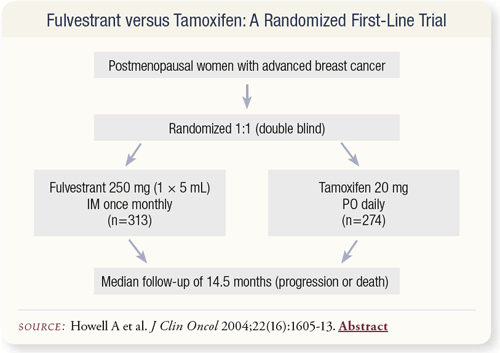
SLIDE 4.15 A recently published trial compared fulvestrant to tamoxifen as first-line therapy for metastatic breast cancer not yet treated with hormones. The median follow-up was about 15 months.
|
| |
|
|
| 4.16 |
|
| |
|
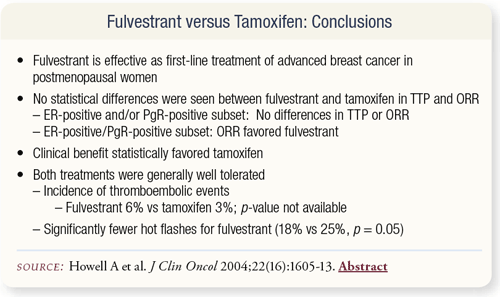
SLIDE 4.16 The trial found a lower incidence of hot flashes with fulvestrant. The two drugs were similar in terms of time to progression and overall response rate.
|
| |
|
|
| 4.17 |
|
| |
|

SLIDE 4.17 Choosing the right sequence of hormonal therapies can be difficult because these agents are essentially equivalent — although perhaps some are slightly better. Data from small trials presented at the San Antonio Breast Cancer Symposium and other meetings can help in clarifying the choices. In a Phase II trial presented in San Antonio, women with metastatic breast cancer all received fulvestrant after progression on tamoxifen and an aromatase inhibitor. Fulvestrant demonstrated a clinical benefit of about 41 percent with very few side effects; hence, fulvestrant has potential activity after tamoxifen and aromatase inhibitors.
|
| |
|
|
| 4.18 |
|
| |
|
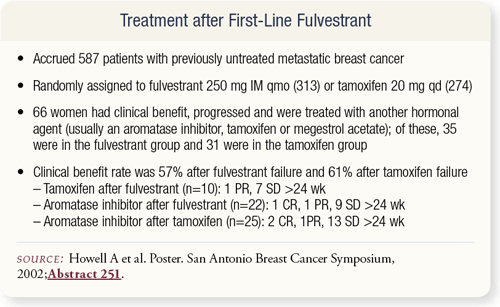
SLIDE 4.18 A more complicated question is whether fulvestrant should be given first line and what happens afterward. In the British study of tamoxifen versus fulvestrant, 66 women had clinical benefit from fulvestrant and then progressed and were treated with another hormonal agent. After fulvestrant failure, the clinical benefit rate was still 57 percent from other agents.
|
| |
|
|
| 4.19 |
|
| |
|

SLIDE 4.19 The implications, in terms of clinical practice, are that fulvestrant and anastrozole are equal after progression on tamoxifen. Should we be using fulvestrant? I think it is an option as first-line treatment despite its expense, because it’s paid for by Medicare. You can give this to someone in your office, as opposed to her paying $300 a month for aromatase inhibitors. Compliance is another consideration — some patients just don’t want to take pills and even though they tell you they’re taking their pills all the time, they really don’t. These are the patients for whom fulvestrant may be appropriate instead of an aromatase inhibitor. Data from small, Phase II studies suggest we can give fulvestrant after failure of other hormonal agents.
|
| |
|
|
| 4.20 |
|
| |
|

SLIDE 4.20 What are some options for sequencing endocrine therapies? One option is first-line tamoxifen followed by fulvestrant, an aromatase inhibitor or inactivator, and then megesterol. A second option is first-line tamoxifen followed by an aromatase inhibitor, fulvestrant and then megesterol. Or an aromatase inhibitor and then steroidal aromatase inhibitor, fulvestrant and megesterol. Many of us start with an aromatase inhibitor in postmenopausal women and then either fulvestrant or tamoxifen followed by either megesterol and tamoxifen or fulvestrant and megesterol. Many options are available. Clinically, if a woman has responded to one hormonal agent for a while — whether it’s months or years — she will likely respond to another one later on.
|
| |
|
|
| 4.21 |
|
| |
|
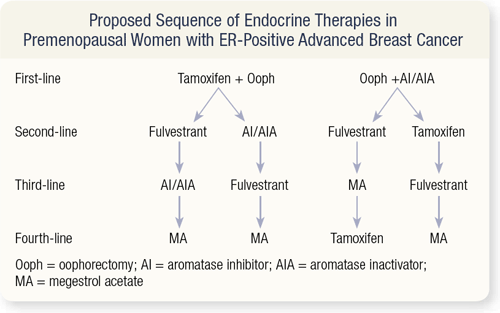
SLIDE 4.21 In premenopausal women, aromatase inhibitors as monotherapy should not be utilized. Aromatase inhibitors given to a premenopausal woman decrease the peripheral estrogen, signaling the ovary to increase estrogen production. This is the complete opposite of the desired effect.
I have seen premenopausal women with metastatic breast cancer treated up front with an aromatase inhibitor who have come to me because they had a flare of their disease and no one knew what to do. The simple thing to do was an oophorectomy.
Data from Europe suggest that the combination of tamoxifen and oophorectomy is probably superior to oophorectomy alone. Then one could continue with fulvestrant or an aromatase inhibitor or inactivator. If the patient progresses on fulvestrant, I would give an aromatase inhibitor or inactivator. At that point, if they progress on an aromatase inhibitor, I’d give fulvestrant, and then finally, megesterol.
The alternative first-line therapy is oophorectomy and an aromatase inhibitor or inactivator. Some antagonism may occur between the LHRH agonist (if the oophorectomy is nonsurgical) and an aromatase inhibitor or inactivator. Some patients who have rising tumor markers or develop symptoms (or both) when treated with this combination have been found to still have premenopausal estrogen levels. In that situation, surgical oophorectomy followed by an aromatase inhibitor is suggested. After that, the sequence is fulvestrant, tamoxifen or megesterol.
|
| |
|
|
| 4.22 |
|
| |
|

SLIDE 4.22 To summarize the hormonal therapies in postmenopausal women with metastatic breast cancer, I think aromatase inhibitors are superior to megesterol as second-line therapy and probably superior to tamoxifen as first-line therapy. Fulvestrant is likely equal to aromatase inhibitors and tamoxifen as first- or second-line hormonal therapy for metastatic breast cancer. Third- and fourth-line responses to hormonal agents are not uncommon.
|
| |
|
|
| 4.23 |
|
| |
|

SLIDE 4.23 I’ve talked about the past; now I’d like to briefly talk about the future — using cellular assays to predict response and survival in women with metastatic breast cancer.
|
| |
|
|
| 4.26 |
|
| |
|
SLIDES 4.24 - 4.26 In a prospective trial sponsored by the Immunicon Corporation and spearheaded by Dan Hayes at the University of Michigan and colleagues from MD Anderson, the Cleveland Clinic, Duke University and the University of Arizona, a device developed by the Immunicon Corporation was clinically applied to measure circulating tumor cells and tumor cell burden. Their hypothesis was that such measurements could help predict whether a woman would relapse or progress on systemic therapy for metastatic breast cancer. The study participants were women with progressive, measurable, metastatic breast cancer who were starting a new systemic therapy. These women had a good ECOG performance status (0-2) and, therefore, were expected to live a reasonable amount of time.
|
| |
|
|
| 4.27 |
|
| |
|
SLIDE 4.27 A brief explanation of the cell-counting procedure will help in understanding the concept of measuring the tumor cells. The patients had 7.5 cc’s of blood drawn in a CellSave® tube, which uses a proprietary preservative that allows the cells to be immunohistochemically stained.
|
| |
|
|
| 4.29 |
|
| |
|

SLIDE 4.28 Circulating tumor cells (CTC) can be easily read by antibody staining. An automated machine, the CellSpotter®, is used to do the reading. A cartridge is placed into the scanning microscope, which will scan the entire scope to identify potential circulating tumor cell candidates, which are then given to a technician or a pathologist to read.
SLIDE 4.29 The results of the Immunicon study were published in the New England Journal of Medicine. They initially did a training set to establish the number of circulating tumor cells that would best differentiate patients who will progress early from patients who will progress late. They found that the number of cells reached a plateau at about five. Applying this five circulating tumor cell cut-off, they noted that patients with less than five circulating tumor cells in their 7.5-cc blood sample had a progression-free survival of about 7.3 months. Patients with five or greater circulating tumor cells had a progression-free survival of a little less than three months. A doubling of progression-free survival occurred with less than five circulating tumor cells at baseline. In a validation set, the researchers drew blood from another 75 randomly selected patients. In the validation set, the progression-free survival of patients who had five or more circulating tumor cells was about 2.4 months; patients with less than five had progression-free survival of about 6.7 months.
|
| |
|
|
| 4.30 |
|
| |
|
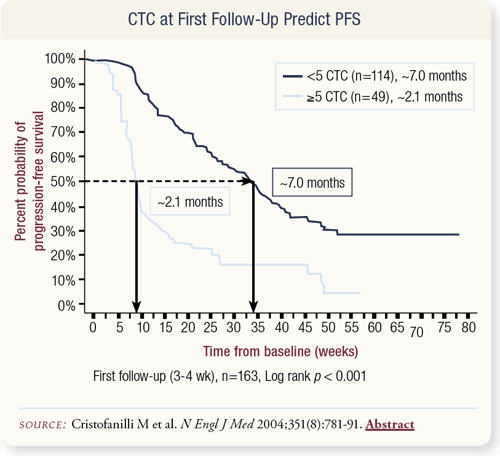
SLIDES 4.30 Ideally, we would like to not only predict progressionfree survival at baseline but also be able to predict whether therapy affected this progression-free survival. To test whether this was viable, the Hayes group drew blood at various intervals throughout therapy.
They found that at about one month after the initiation of new systemic therapy, patients with greater than five circulating tumor cells had a progression-free survival rate of 2.1 months, suggesting that their initial therapy was probably not going to work. However, participants with five or less circulating tumor cells had a progression-free survival rate of about seven months.
|
| |
|
|
| 4.32 |
|
| |
|
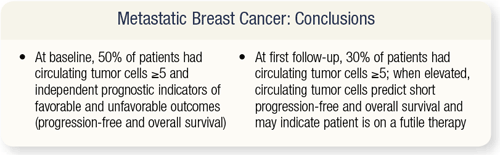
SLIDE 4.31 In a multivariate analysis of all the factors that went into determining progression-free survival and overall survival, one of the best predictors for progression-free survival was less than five circulating tumor cells. Patients with less than five circulating tumor cells at baseline had about 75 percent improvement in progression-free survival. At first follow-up, less than five circulating tumor cells after therapy seemed to predict for a very good progression-free and overall survival.
SLIDE 4.32 From this cell count study, researchers concluded that in metastatic breast cancer, 50 percent or more of patients have greater than five circulating tumor cells at baseline. These circulating tumor cells are an independent prognostic indicator for favorable and unfavorable outcomes. At first follow-up, 30 percent of patients had five or greater circulating tumor cells, so 20 percent of the patients derived benefit from chemotherapy or hormonal therapy.
But in patients in whom the tumor cells were still elevated, it predicted a short progression-free and overall survival, and potentially indicated that therapy should be changed. Not only do we have clinical judgment and other parameters, but we also may have this independent assay to consider. These findings need to be validated in larger studies but in the future will likely prove to be a good adjunct to standard staging modalities for us to predict recurrence.
|
| |
|
|
| 4.33 |
|
| |
|
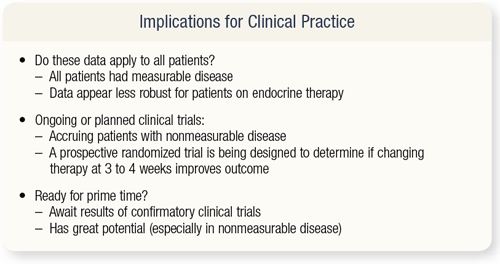
SLIDES 4.33 What are the implications of a cell-counting tool in clinical practice? The patients studied by Hayes and colleagues had measurable disease, but the majority of patients with metastatic breast cancer have nonmeasurable disease (evaluable disease). Trials are now accruing patients with nonmeasurable disease to determine whether these data will be as robust for patients on endocrine therapy alone.
Dan Hayes is organizing a prospective randomized trial, called the BRATS study, designed to determine if a change in therapy after three to four weeks, based on a circulating tumor cell assay, will improve a patient’s outcome. Patients who have five circulating tumor cells after a month of therapy will be randomly assigned to a change of therapy or continued therapy. As I said before, we need to await the results of confirmatory clinical trials, but I’m presenting this now because it has great potential for the future.
|
| |
|
|
|
|
|
|
