| You are here: Home: BCU 8|2004: Aman U Buzdar, MD
Aman U Buzdar, MD |
EDITED COMMENTS |
 MD Anderson trial neoadjuvant/ adjuvant chemotherapy trial MD Anderson trial neoadjuvant/ adjuvant chemotherapy trial
We currently have a trial evaluating the role of capecitabine/docetaxel in the adjuvant and neoadjuvant settings. All patients entering the trial with intact primary tumors are randomly assigned to receive either FEC  paclitaxel or FEC paclitaxel or FEC  capecitabine/docetaxel in the neoadjuvant setting. Patients who have previously undergone surgery receive the same randomized treatment, but they receive it in the adjuvant setting. capecitabine/docetaxel in the neoadjuvant setting. Patients who have previously undergone surgery receive the same randomized treatment, but they receive it in the adjuvant setting.
The control arm is very similar to the control arm we used in our neoadjuvant trastuzumab study. The only difference is that we are using weekly versus every three-week paclitaxel for 12 weeks. The final endpoint will combine the neoadjuvant and adjuvant subgroup data, and evaluate disease-free and overall survival. The neoadjuvant group has an advantage in that we will be able to find the clinical complete remission rate, the pathological complete remission rate and a number of other endpoints.
We currently have more than 200 patients enrolled in the study. In the first cohort, we gave a somewhat higher dose of capecitabine and saw an increase in morbidity. We reduced the dose of capecitabine and, with the use of this attenuated dose, we are seeing more acceptable toxicity.
Now the big question remains: What is the long-term and short-term efficacy? The data are continuously being monitored but we really won’t have any information until we have enough patients in the neoadjuvant setting to determine whether the regimens are similar or one is better than the other.
MD Anderson neoadjuvant trial of trastuzumab
At MD Anderson we believe that if a patient with an intact primary tumor is going to need systemic chemotherapy, unless the tumor is small, it is better for them to receive it in the neoadjuvant setting where we can tell whether the treatment is having an impact on the disease or if it is just causing toxicity. A few years ago we launched a study designed to accrue 164 patients with HER2- positive disease and intact primary tumors.
All of the patients enrolled in the trial received four courses of every three-week paclitaxel followed by 12 weeks of FEC (2.1). We used epirubicin instead of doxorubicin because it has a better cardiac safety profile. One half of these patients also received weekly trastuzumab for 24 weeks. Every patient had a baseline cardiac scan and then repeat scans at 12 and 24 weeks.
In our previous experience with this chemotherapeutic regimen, about 21 percent of unselected patients had pathological complete remissions. Pathological complete remission is defined as having no tumor left in the breast or in the lymph nodes after therapy. We were hoping that the addition of trastuzumab to chemotherapy would elevate the pathological complete response rate from 21 percent to 41 percent — a 20 percent improvement.
The trial was interesting because we knew what the pathological outcome was as soon as the patient completed surgery. As soon as we had results from 34 patients, we were able to see that 65 percent of the patients in the trastuzumab arm had no tumor whereas only 25 percent of the patients who received chemotherapy alone were tumor free. This was much higher than we had anticipated or hoped for. The clinical response rate was even more striking, as 87 percent of the patients had clinical complete remission in the trastuzumab arm compared to about 50 percent in the chemotherapy-alone arm.
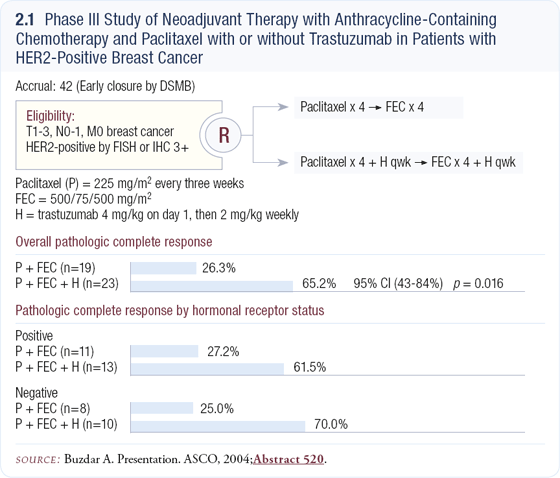
We discussed these data with our institutional Data Monitoring Committee, which looked at them independently and came to the conclusion that the findings were so striking that even if we continued the trial to reach accrual, the results would be similar. Thus the trial was stopped early.
Cardiac safety in neoadjuvant trastuzumab study
Anthracycline-based regimens are the best combination chemotherapies we know. They also have significant synergy with trastuzumab in the metastatic setting. For these reasons, we wanted to test an anthracycline/trastuzumab combination in the neoadjuvant setting; however, the question has always been the cardiac safety data.
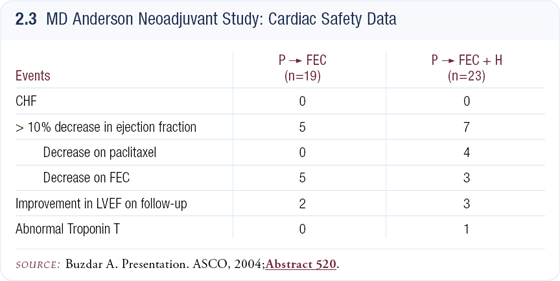
We debated the design of a trial that would allow us to accomplish this goal, and the conclusion we came to was to use a safer anthracycline and to use it at a limited dose. All patients in this trial received 75 mg/m2 of epirubicin in each of four cycles. No patients in the trial had any type of clinical cardiac dysfunction.
We observed a slightly increased incidence of reduced ejection fractions in patients enrolled in the trastuzumab arm compared to the patients in the chemotherapy- alone arm. All of these changes were observed on cardiac scan. What was also surprising was that in almost all of the patients who had drops in their cardiac ejection fractions, the LVEFs returned to normal after therapy was completed.
We also measured troponin T levels in all patients enrolled in the trial. A recent paper in the New England Journal of Medicine reports on patients being treated with doxorubicin for leukemia (2.2). In that trial, troponin T levels were evaluated for patients receiving doxorubicin alone and doxorubicin with dexrazoxane to see whether that agent can protect cardiac function.
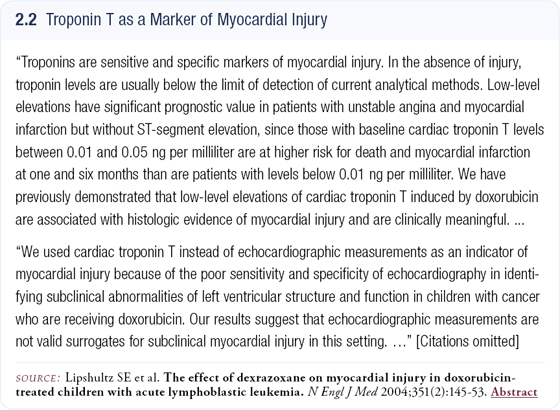
Patients receiving dexrazoxane in addition to their chemotherapy had many fewer elevations in their troponin T levels than those receiving doxorubicin alone. The troponin T test is a very sensitive test that can predict whether an anthracycline is causing myocardial damage or not. In all of our patients, with the exception of one on the trastuzumab arm, troponin T remained normal throughout the trial (2.3).
Nonprotocol use of neoadjuvant and adjuvant trastuzumab
Before this data was available, we did not offer neoadjuvant trastuzumab to any patient outside the context of a clinical trial. However, now that the data is in the public domain, I think it is our responsibility to share the information and discuss the issue with our patients. As long as the patient and the physician understand that uncertainties exist regarding the data, the cardiac safety and the long-term outcome, I believe it is a reasonable approach.
At our institution, based on the recommendation of the Data Monitoring Committee, we stopped the control arm of the study. Currently, all patients are being offered chemotherapy with trastuzumab in the neoadjuvant setting. We want to expand our experience, determine whether this data is reproducible and acquire long-term safety data.
On the other hand, if a woman with high-risk node-positive disease comes to MD Anderson seeking adjuvant trastuzumab, our group is divided on the issue. Some physicians within our group believe that a woman at high risk should be offered this therapy in the neoadjuvant or adjuvant setting, whereas others want to be conservative and not offer it.
My experience is that patients who have four or more positive nodes tend to not do well, especially if they have HER2-positive disease. I think we have to discuss these options and let the patients know about these treatments because “the genie is out of the bottle.” After appropriate discussion, if the patient agrees and accepts the uncertainties and the limitations of the available data, I am inclined to offer this therapy.
ATAC trial update
As we speak, the ATAC data is being collected and analyzed. As per the protocol, the results will be made public in December 2004. I anticipate that we will have updated survival and efficacy data with a median follow-up of roughly six years, but I have no idea what the data will show. However, even if no statistically significant survival advantage exists up to that point, we still cannot ignore the disease-free survival advantage and the major safety advantages over tamoxifen.
Two key safety issues must be kept in mind with tamoxifen. One is thromboembolic complications, which are unpredictable, and the other is uterine cancer. We also have to recognize that a handful of women in the prevention trial also developed uterine sarcoma. Just the other day I was searching the literature on PubMed and came across a host of cases reporting uterine sarcoma in women who have been treated with tamoxifen.
Side effects are experienced by all patients treated with tamoxifen, whereas safety advantages for anastrozole are here to stay. I think we cannot ignore the fact that anastrozole is a safer drug and overall has a better therapeutic index than tamoxifen.
Role of aromatase inhibitors in clinical practice
I think it is human nature, especially within the academic community, to be cautious when you only have the results of a single study; however, when additional studies confirm those findings, people are much more willing to accept the data. The data from MA17, Intergroup Exemestane Study and an Italian study have a very consistent message — it doesn’t matter when you use an aromatase inhibitor, it will result in fewer side effects and a prolongation of disease-free survival, which I believe will eventually translate into a survival advantage. The data now clearly demonstrate that you can change the natural history of breast cancer by offering aromatase inhibitors in various patient populations.
Adjuvant bisphophonates to offset aromatase-inhibitor-related bone loss
I am always surprised by the number of patients whom I expect to have normal bone density but are found to be osteopenic or osteoporotic. In a number of these patients, I have started an aromatase inhibitor and also given them a bisphosphonate. On some occasions, within six months to a year, I have seen positive changes in bone density.
I believe bisphosphonates are effective in preventing, reversing and improving bone loss in osteopenic or osteoporotic patients, and that is why I do not make treatment decisions based on a patient’s bone health. If a patient has a risk of recurrence, I believe we should offer them better therapy and use other effective therapies with established value to manage osteopenia or osteoporosis.
In patients who are osteopenic or osteoporotic, I often use alendronate and can tell you from my own experience that several months down the line, a number of these patients will actually have improvements in their bone density in spite of being on an aromatase inhibitor.
Select publications
 |
Dr Buzdar is a Professor of Medicine and Deputy Chairman of the Department of Breast Medical Oncology at the University of Texas MD Anderson Cancer Center in Houston, Texas. |
|
