| You are here: Home: BCU 4|2004: C Kent Osborne, MD
C Kent Osborne, MD |
EDITED COMMENTS |
CAN-NCIC-MA17 trial of letrozole versus placebo after five years of adjuvant tamoxifen
We know from previous studies that continuing adjuvant tamoxifen beyond five years is not beneficial and, according to one study, might even be deleterious. MA17 randomly assigned patients who had completed five years of tamoxifen to five years of letrozole or a placebo.
The trial was stopped early because the estimated benefit of letrozole was substantially greater than expected — unblinding revealed a 40 percent reduction in recurrences in the patients on letrozole.
Today, many women diagnosed with breast cancer receive adjuvant aromatase inhibitors. But for the thousands currently on adjuvant tamoxifen, the results of MA17 are applicable. Women with very low-risk disease may not derive enough benefit from an additional five years of hormonal therapy to make it worthwhile, but for women at higher risk, switching to an aromatase inhibitor is a reasonable alternative and I offer it to my patients.
MA17 limited enrollment to patients who had completed tamoxifen within the past three months, but I'm comfortable extending that to six months or even 12 months for patients at very high risk.
Estrogen deprivation in HER2-positive, ER-positive breast cancer
As predicted based on model systems, estrogen deprivation has been shown to be beneficial in HER2-positive, ER-positive tumors. Even though such tumors have numerous estrogen receptors in the membrane and nucleus, and high growth factor signaling, if the estrogen receptors are not activated with a ligand such as tamoxifen or estrogen, neither of these pathways are activated.
A few years ago people doubted the results of Matt Ellis' study in which letrozole produced a much higher response rate than tamoxifen in patients with HER2-positive disease because the study involved a small number of patients. However, Mitch Dowsett's IMPACT trial has shown that another aromatase inhibitor — anastrozole — is also much better than tamoxifen in these patients. In practice, when HER2 is overexpressed, estrogen deprivation may be a better choice than tamoxifen — either an oophorectomy in younger women or an aromatase inhibitor in older women.
Tamoxifen resistance and the conversion of tumors from HER2-negative to HER2-positive
We have laboratory and clinical data suggesting that tamoxifen can convert a tumor from HER2-negative to HER2-positive (Figure 3.1). In an in vivo model using a cell line with low EGFR and HER2, we've shown that initially, tamoxifen has antiestrogenic activity on the tumor. However, after three or four months tamoxifen resistance develops, and tamoxifen acquires the ability to stimulate the tumor.
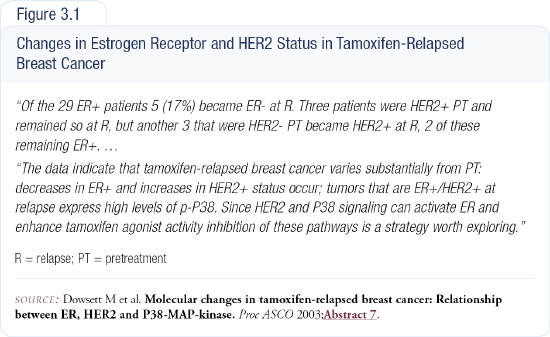
At three or four months, increased EGF and HER2 receptors are on the cell membrane. Blocking those can prevent the development of tamoxifen resistance. A tyrosine kinase inhibitor like gefitinib, trastuzumab or both can be used to inhibit the EGFR/HER2 pathway. The combination is superior because each drug inhibits the growth factor pathway in a different way.
Clinical data demonstrating the conversion of tumors from HER2-negative to HER-2 positive is limited because it requires serial biopsies. We collaborated on a study with Mitch Dowsett in which we had biopsies from 37 patients taken just before tamoxifen use, with subsequent biopsies taken at the time of tamoxifen resistance. We found three cases that were initially HER2-negative but converted to positive at the time of tamoxifen resistance. Two of those were actually gene-amplified.
We need to confirm that with a larger data set, but it may be that in some patients, EGFR and HER2 are upregulated at the time of tamoxifen resistance. From a practical perspective, patients with HER2-negative tumors who progress on tamoxifen should be retested because if the metastases are positive, trastuzumab may be indicated.
As we develop more targeted therapies, we'll need to perform more biopsies in the metastatic setting to identify changes in the tumor profile in order to select the appropriate therapy. EGFR and HER2 can go up in a small proportion, estrogen receptor goes away in 15 to 20 percent, and there may be other potentially targeted molecules that can change over time.
Mechanism of action of fulvestrant
Fulvestrant degrades the estrogen receptor — it's a complete antagonist on the nuclear receptors, and studies suggest it is also an antagonist on the membrane receptors. This may explain why HER2-overexpressing tumors respond well to fulvestrant in cultured model systems and in vivo models. Hypothetically, fulvestrant in the HER2-overexpressing tumor blocks the cross-talk by eliminating the estrogen receptor; thus the estrogen receptor can't activate the growth factor pathway. We don't have clinical data to confirm this, but we expect that in patients with ER-positive, HER2-positive tumors, fulvestrant might be more effective than tamoxifen.
Prolonged duration of response with fulvestrant
In animal models comparing fulvestrant to tamoxifen, fulvestrant substantially prolonged the time it took for the development of resistance. However, at the current dose we have not yet seen that translate into a longer duration of response in clinical trials. On the other hand, in the two trials of second-line fulvestrant versus anastrozole, the duration of response with fulvestrant was significantly longer in one of the trials — 19 months versus 11 months — and the combined analysis showed a longer duration of remission in patients treated with fulves-trant (Figure 3.2). Although clinical data suggest that long remissions can be seen with fulvestrant, I believe we haven't had enough studies with fulvestrant and we don't know the optimal dose.
Efficacy of fulvestrant in the metastatic setting
In patients progressing on tamoxifen, tamoxifen binds the estrogen receptors and may actually stimulate growth of the tumor it certainly is no longer inhibiting it. Treating these patients with an aromatase inhibitor will be ineffective until all the tamoxifen is gone, which takes a couple of months.
Fulvestrant, on the other hand, competes with tamoxifen for binding, thus the response may be quicker with fulvestrant than with an aromatase inhibitor in that setting.

We expected fulvestrant to be superior to tamoxifen, but in the first-line setting it proved to be similar, not better. That’s peculiar because second-line trials show fulvestrant to be equal to or better than aromatase inhibitors, and aromatase inhibitors have been shown to be superior to tamoxifen.
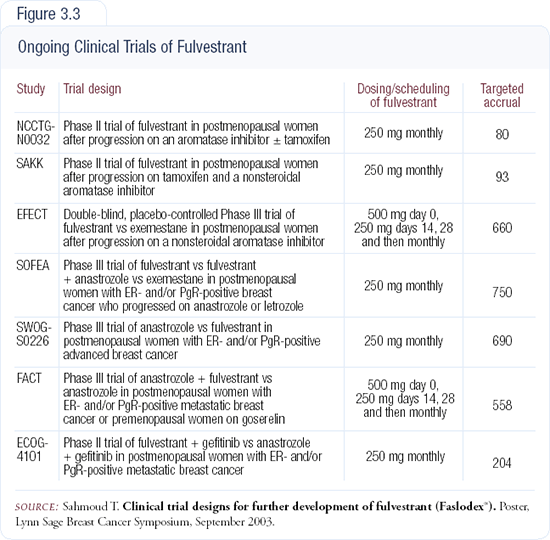
It may be that we’re just not dosing fulvestrant correctly. We know from the randomized trial that half of the currently recommended dose is insufficient, and we know it takes three to six treatments to achieve steady state blood levels with fulvestrant, so perhaps a higher dose or a loading dose (or both) is required. These options are being investigated (Figure 3.3).
Adjuvant trastuzumab
I’m optimistic that trastuzumab will be effective in targeting HER2-overexpressing tumors in the adjuvant setting. With tamoxifen, our first targeted therapy, we saw a 30 percent response rate and 20 percent stable disease rate in patients with ER-positive metastatic disease. When used for five years in the adjuvant setting, however, it reduced the recurrence rate by almost half. In HER2-positive metastatic disease, approximately 25 percent of patients experience remission with trastuzumab monotherapy, but what if trastuzumab offers an advantage similar to that of tamoxifen in the adjuvant setting?
It's clear that when you're treating widespread metastatic disease, all of these therapies, particularly chemotherapy and targeted therapies, have a much greater effect on micrometastases than macrometastases. We'll know more in a year or two when we begin receiving data from the current adjuvant trastuzumab trials (Figure 3.4).
Neoadjuvant trastuzumab monotherapy
Jenny Chang, a member of our group, presented a study of neoadjuvant trastu-zumab given weekly for three weeks to women with HER2-overexpressing tumors. Within three weeks, 26 percent of the patients already had a partial remission, and all of the patients had some reduction in tumor size. I think these targeted therapies will be much more effective early on in the tumor's life than in treating widespread metastatic disease. And it may turn out that we'll see definite activity of these agents, even with just a few weeks of treatment.
The study had only 27 patients and the objective was to learn about predictors of trastuzumab activity, what targets are being blocked by trastuzumab and so forth. But, to our surprise, within three weeks we saw these responses. The median reduction in tumor volume was 20 percent.

Now we're beginning to think that maybe we should do a trial of longer-term treatment. What if we treated for six weeks rather than three? Maybe the majority of the patients would already have a partial remission. Plus, we learned that trastuzumab induces apoptosis and doesn't seem to have much of an impact on cell proliferation, which was shown in vitro (Figure 3.5).
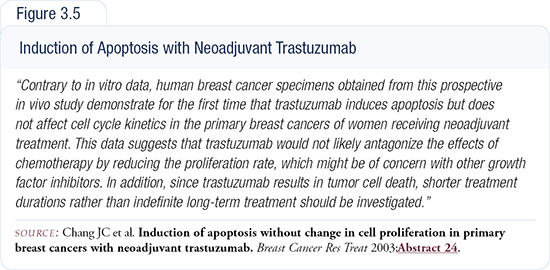
But tumor cells growing on glass in vitro don’t mimic the in vivo situation very well. You can obtain some clues but — unlike receptor tyrosine kinase inhibitors like gefitinib, which inhibit cell proliferation — trastuzumab seems to work by apoptosis, at least in this one study. It induces apoptosis within a week of treatment.
Obviously, it was a small study with a very short duration, but we learned a great deal about trastuzumab. We saw apoptosis and rapid reductions in tumor size in some patients, and none of the patients progressed during that threeweek period. People had thought, based on other trials, that trastuzumab would downregulate and degrade HER2, but we didn’t observe a change in the HER2 level during that period of time.
A great deal of interesting biologic information was derived from this small study that will help plan subsequent neoadjuvant studies to determine how these drugs should be used.
Select Publications
 |
| Dr Osborne is the Director of the Breast Center and Professor of Medicine and Cellular Biology at Baylor College of Medicine in Houston, Texas. |
|
