| You are here: Home: BCU Surgeons 2004 Vol 3 Issue 3: Nicholas J Robert, MD
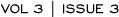
Nicholas J Robert, MD |
EDITED COMMENTS |
 Implications of the ATAC trial data Implications of the ATAC trial data
The ATAC data make a strong case to use an aromatase inhibitor in the adjuvant setting. In this trial of more than 9,000 patients, anastrozole demonstrated approximately a 20 percent proportional improvement in diseasefree survival compared to tamoxifen, and had a more favorable toxicity profile. Anastrozole is associated with less risk of thromboembolic disease and uterine cancer. While the loss of bone density is greater with anastrozole, even postmenopausal women on tamoxifen are at risk for bone loss and osteoporosis. I believe the oncology community is becoming more aggressive in evaluating and treating this toxicity and, fortunately, we have agents to manage bone loss.
Aromatase inhibitors versus tamoxifen in the adjuvant setting
Over the past couple of decades, tamoxifen has had a huge impact on the management of breast cancer, but its use in the adjuvant setting may be declining. Several studies have demonstrated the superiority of aromatase inhibitors over tamoxifen, including the ATAC trial, the NCIC-CAN-MA17 trial in which women received letrozole after five years of tamoxifen, and two trials in which women were switched to an aromatase inhibitor after two or three years of tamoxifen. The Intergroup study utilizing exemestane and Boccardo's trial utilizing anastrozole demonstrated an advantage to switching early from tamoxifen to the aromatase inhibitor.
When I use endocrine therapy in newly diagnosed patients, I use anastrozole. If I'm going to switch therapy after two or three years of tamoxifen, I use exemes-tane, but after five years of tamoxifen, I choose letrozole.
Risk of recurrence after five years of adjuvant tamoxifen
In an article published in the Journal of Clinical Oncology in 1996, Dr Saphner et al reviewed trials from the ECOG database to determine annual hazard rates of recurrence for breast cancer after primary therapy (Figure 1.1). Patients with four or more positive nodes had a higher risk of recurrence in all time intervals.
I believe nodal involvement is key to the risk of recurrence after the first five years. Letrozole is appropriate in a patient with node-positive breast cancer who completed five years of tamoxifen a year or two ago, but if four or five years have passed and the patient had a small tumor and node-negative disease, the benefit of letrozole would be marginal.
One issue raised by the MA17 and ATAC trials is the selection of endpoints in adjuvant studies. These trials included contralateral tumors and local and regional recurrences. In the future, I suspect we'll be more interested in the distant disease recurrence endpoint. If we had used that as the endpoint in the MA17 trial, the study would probably still be open and we may have obtained additional information.
Assessment of HER2 status and choice of therapy
To determine a patient's HER2 status, FISH is currently the best method we have in terms of linking outcome with intervention. I believe ascertaining the HER2 status in patients with metastatic breast cancer is mandatory. One can use the primary tissue; however, whenever feasible, one should biopsy metastatic lesions and re-evaluate the HER2 and hormone receptors.
In the adjuvant setting, establishing the patient's HER2 status is important for several reasons. Circumstantial evidence suggests anthracycline-containing regimens are more effective than non-anthracycline regimens in treating HER2-positive tumors. The HER2 status may also affect one's choice of endocrine agents, but again, this may become academic as the enthusiasm for aromatase inhibitors increases. Another reason to evaluate the HER2 status is its prognostic value. Most oncologists believe HER2-positive disease is more aggressive and the patients may have a greater risk of recurrence.
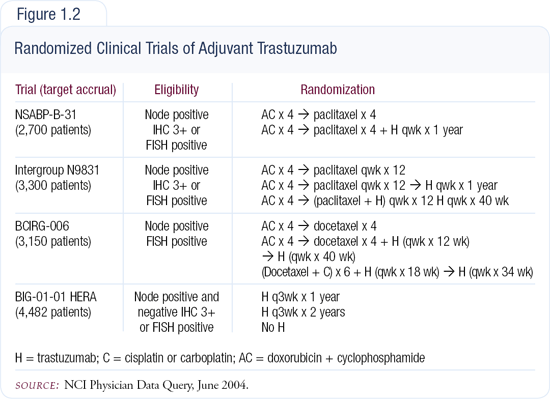
Clinical trials evaluating adjuvant trastuzumab
Four adjuvant trastuzumab trials have been initiated to test three different principles (Figure 1.2). Two ongoing studies — the NSABP and Intergroup trials — basically compare the gold standard, doxorubicin/cyclophosphamide followed by a taxane, with or without trastuzumab. This is a reasonable "next-step" type of protocol. In the completed BCIRG-006 trial, two arms were similar to these trials, but the third arm — docetaxel/carboplatin/trastuzumab — was based on exciting preclinical and clinical work that showed the addition of carbo-platin improved outcome. The HERA trial is evaluating the duration of adjuvant trastuzumab, randomly assigning some patients to one or two years of therapy. In the other three trials trastuzumab is given for one year, but no data exist to suggest that's the optimal duration. The results will be interesting because if we use trastuzumab like we use endocrine agents, we may be looking at very prolonged usage.
Treatment of patients with HER2-positive, ER-positive metastases
HER2-positive Stage IV disease encompasses a heterogeneous group of patients who have different rates of progression. In a patient with relatively indolent HER2-positive, ER-positive disease who has received tamoxifen, I believe it’s appropriate to consider another endocrine intervention. However, in a patient whose disease is more life-threatening, I believe it's reasonable to consider an endocrine intervention plus trastuzumab.
A trial evaluating anastrozole with or without trastuzumab is nearing completion, but we don't have the data yet. HER2-positive tumors may respond better to aromatase inhibitors than to tamoxifen, which I believe relates to some interaction downstream from the estrogen receptor. Tamoxifen binds the estrogen receptor, but HER2-positive tumors have an alternate pathway by which the receptor can be activated. Aromatase inhibitors basically eliminate estrogen and thus avoid its activation.
Role of the surgeon in requesting tumor markers
Generally the surgeon is involved in the initial diagnosis, but often today it's the radiologist who performs a stereotactic biopsy, and the specimen is sent to the pathologist. The pathologists must not only evaluate the histology, but also evaluate hormone receptors and HER2 status. These studies are probably even more important than studies of the proliferation index, because we can usually capture that with grade. So the pathologist has a very important role.
It is frustrating to be involved in a case in which the surgeon never requested these studies. When you see the patient two or three weeks after surgery, you have to wait another one to two weeks for that information before you can provide some intelligent advice about their treatment options.
It's important for these studies to be performed up front. Breast cancer care is a multidisciplinary process. It's not necessary to have a breast cancer center, but you certainly should have the treatment team interacting and have some general principles about how to evaluate patients.
Select publications
 |
Dr Robert is Chairman of the Research Committee at the Cancer Center of the Inova and Chair of the Breast Cancer Committee of the US Oncology Research Network in Fairfax, Virginia. |
|
