| You are here: Home: BCU 3|2004: Daniel R Budman, MD, FACP
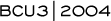

 |
Edited comments by
Daniel R Budman, MD, FACP |
 |
Impact of dose reduction on clinical outcome
Dr Lyman’s data evaluating delivery of full-dose therapy included tens of thousands of women treated for breast cancer in clinical practices, and evaluated all the permutations of the regimens we currently use. It revealed that over 60 percent of women are not receiving full-dose therapy, which is a major concern because most anticancer drugs have a narrow dose-response curve, so there’s a narrow therapeutic index at the upper limits of the conventional dose range.
Several years ago, data from CALGB-8541 demonstrated that in the adjuvant setting, full-dose conventional-range therapy was significantly better in the treatment of node-positive breast cancer (Figure 3.1). The study examined three cohorts of patients, each receiving different doses of CAF, and evaluated the dose delivery and the total cumulative dose. Patients receiving the higher doses experienced a marked statistical improvement over the observation period in both disease-free and overall survival in all subsets, and that has continued 10 years later. There was a steep dose-response curve, so we’ve learned that compromising dose, either initially because of other conditions or reducing dose later, can be detrimental to outcome.
Anthracycline dose-response curves
There’s evidence of a dose-response in several studies comparing anthracycline doses. Craig Henderson’s study published in the Journal of Clinical Oncology evaluated three doses of doxorubicin — 60, 75, and 90 mg/m2 — combined with cyclophosphamide 600 mg/m2 with or without subsequent paclitaxel. Doxorubicin doses above 60 mg/m2 added nothing but toxicity and the dose-response curve suggests doses below 60 mg/m2 are detrimental. In the FEC trials, epirubicin 100 mg/m2 was better than 75 or 50 mg/m2 and there was dose-response as well.
Capecitabine in the metastatic breast cancer setting
When I see a patient who received adjuvant anthracyclines and taxanes for metastatic disease, I generally use an oral agent such as capecitabine. We know capecitabine is efficacious in that setting and quality of life is improved. I haven’t seen much hand-foot syndrome in my patients with the appropriate dosing. Depending on the patient’s age and renal function, I use between 750 and 1,000 mg/m2 twice daily for 14 out of 21 days. One concern with capecitabine is dihydropyrimidine dehydrogenase (DPD) deficiency, but that occurs in only one out of 1,000 patients.
The dosing of capecitabine is controversial. Because it has a broad therapeutic index, I believe we can utilize lower doses and maintain efficacy with little toxicity. In the capecitabine/docetaxel (XT) trial, dose reduction from 2,500 mg/m2 total daily dose to half that dose still retained efficacy. I find the original dose utilized in the Phase I trial — 1,000 to 1,300 mg/m 2 total daily dose —particularly interesting as chronic treatment, and that has not been adequately explored. The two-week on, one-week off schedule was based on European data with small numbers of patients, which showed you could deliver more drug that way, but that doesn’t necessarily mean it’s better. We know in the preclinical models, capecitabine also has antiangiogenic properties, so it may be useful to look again at the dose and schedule.
Clinical trials of capecitabine for metastatic disease
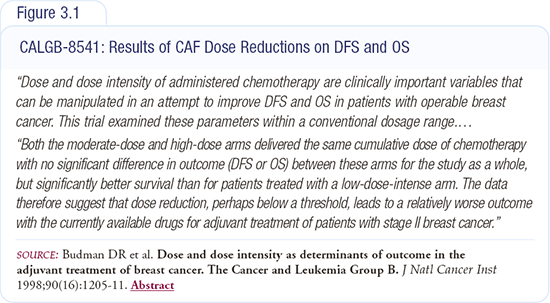
Excluding 5-FU from Henderson’s trial of doxorubicin/cyclophosphamide was purely empirical — no one really knows whether it adds efficacy or just toxicity. There is research underway integrating capecitabine with these regimens in the metastatic setting. We know capecitabine is an active drug. In the first-line setting for metastatic cancer, it’s equivalent to CMF, and in heavily pretreated patients who failed anthracyclines and taxanes, it results in a 30 percent response rate (Figure 3.2).
Taxanes are particularly active, and Nabholtz’s trial showed that AT is better than AC in the metastatic setting. Additionally, ET has been shown to be better than EC, and perhaps we can eliminate the alkylating agents. These combinations are being studied in Europe. A randomized trial is comparing docetaxel/epirubicin/ capecitabine (TEX) versus docetaxel/epirubicin. In Phase II trials, this triplet was very active and if the biochemical evidence of the upregulation of thymidine phosphorylase by capecitabine is clinically significant, one would expect the triplet to be superior in this trial.
Clinical trials of capecitabine in the adjuvant setting
Capecitabine is an obvious choice to study in the adjuvant setting. I’m most interested in Hyman Muss’ Intergroup study comparing capecitabine versus AC or CMF in women over age 65. Based on the chemistry of capecitabine, it wouldn’t surprise me if it proves to be equivalent in efficacy with a superior toxicity profile. In addition, it has the advantage of being an oral regimen. US Oncology and MD Anderson each have adjuvant studies evaluating the combination of capecitabine and docetaxel, but these trials are not mature and it will be some time before we know the results.
Evaluating strategies combining biologic agents with chemotherapy
We believe it’s important to study signal transduction inhibitors combined with chemotherapy. We’re particularly interested in using small molecules to block EGFR function. To that end, we are planning a trial combining a dual kinase inhibitor with capecitabine. Our tissue culture experiments showed marked synergy between these two agents. A French group has published data from a head-and-neck model showing that the tyrosine kinase inhibitors of EGFR upregulate thymidine phosphorylase, which could be the rationale behind that synergy.
Limitations in utilizing body surface area to calculate dose
We have dosed patients by “per meter squared (m2)” since the 1960s, but this was based on the erroneous belief that utilizing body surface area (BSA) normalized dosing between people — the larger the person, the larger the dose (Figure 3.3). We know now that BSA has very little meaning; the important considerations are how the individual absorbs or metabolizes the drug, and metabolism varies tremendously.
Obese patients raise particular concern. The CALGB examined this issue across adjuvant treatments and recommended we not dose-reduce even the morbidly obese patient. I must admit most of my morbidly obese patients have comorbid conditions, such as hypertension, diabetes, etcetera, and I am reticent to administer large doses of cytotoxic drugs to such patients. So, despite the CALGB’s recommendation, I cap the body surface area at 2.0 m2.

Sequencing hormonal agents in the metastatic setting
One of the burning issues in breast cancer today is how best to integrate the various hormonal therapies. We now have a panoply of hormonal therapies available: antiestrogens, aromatase inhibitors, a pure antiestrogen that knocks out the estrogen receptor, and the old progestins. I suspect we’ll shuffle between these agents once we have a better understanding of cell phenotypes. Then we’ll be able to identify the appropriate hormonal therapy for each patient and tailor our treatment before we see actual clinical resistance.
In the metastatic setting, I generally use an aromatase inhibitor first, then an antiestrogen and then fulvestrant. Unless there’s a contraindication, I begin with aromatase inhibitors because I believe there’s sufficient evidence that they are better than tamoxifen for front-line therapy in metastatic disease. I see approximately a 10 percent incidence of articular complaints with aromatase inhibitors, but I’ve found that switching the structure, from a nonsteroidal to a steroidal aromatase inhibitor or vice versa, seems to diminish those complaints.
Fulvestrant in the metastatic setting
Fulvestrant is an active drug and it’s been shown to be equivalent to anastrozole, but we don’t know where to sequence it. In elderly women, there’s a higher incidence of estrogen receptor-positive breast cancer, but there’s no way to know if these elderly patients are reliably taking their oral hormonal agents. In this setting, fulvestrant is an ideal drug because you don’t have to worry about compliance. The responses I’ve seen to fulvestrant have been mainly in this population and I assume that patients who respond after failing an oral hormonal agent do so because of the activity of the fulvestrant, although I can’t be certain that some of it isn’t a compliance issue with the oral therapy.
Fulvestrant can’t be absorbed orally, so it requires injections. Five cubic centimeters has been considered the standard maximum volume that one should inject, but we don’t actually know whether 250 mg is the appropriate dose. Fulvestrant trials comparing 125 to 250 mg showed the higher dose was better, but we don’t know whether an even higher dose would be more efficacious. It’s frustrating that we really don’t know the limits of fulvestrant’s dose-response curve.
The injection itself is not a problem for the motivated patient, but the visits can be a problem for patients who have to rely on others to get to their appointments, as is often the case with the elderly. The majority of patients find it supportive and reassuring to be seen on a regular basis for their treatment, however there are some patients who try to deny their disease and become more agitated by the treatment visits.
Select publications
|
| Dr Budman is Associate Chief of the Don Monti Division of Medical Oncology and Division of Hematology at North Shore University Hospital and Professor of Medicine at New York University in Manhasset, New York. |
|
