| You are here: Home: BCU 1|2004: Harold J Burstein, MD, PhD

| Harold J Burstein, MD, PhD |
Edited comments by Dr Burstein
CAN-NCIC-MA17 trial: Efficacy of aromatase inhibitor following five years of adjuvant tamoxifen
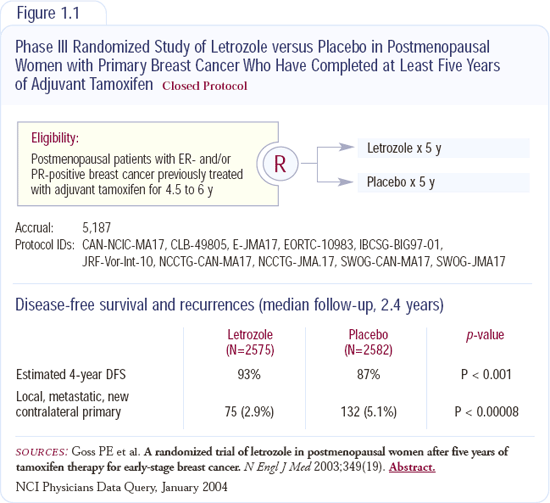
Led by the National Cancer Institute of Canada, MA17 randomly assigned over 5,000 post-menopausal women who had received tamoxifen for between four and a half and six years and were free of tumor, to receive letrozole or a placebo.
Letrozole reduced the rate of breast cancer events by about 50 percent, including the risk of distant metastases and the risk of ipsilateral or contralateral breast cancer. The differences were so robust after only two and a half years that the study was closed before completing its planned five-year duration (Figure 1.1).
The data are exciting because letrozole has the potential to improve the long-term prognosis for the largest demographic group of patients - postmenopausal women with hormone receptor-positive breast cancer. Historically, these women have been offered five years of tamoxifen; now many such patients should consider taking letrozole after completing that therapy.
It's always exciting to close a study early because of such good news, but follow-up trials are needed to address unanswered questions about the best way to use letrozole in this setting. Also, there are concerns regarding the profound estrogen deprivation effects of aromatase inhibitors, particularly osteoporosis. We can study those issues, and there are interventions, but it means that we have to pause before blindly recommending this therapy to everyone.
Risk of recurrence after adjuvant tamoxifen
A patient's risk of breast cancer recurrence is greatest the first few years after diagnosis; after three or four years it begins to plateau. While this is particularly true for hormone receptor-negative breast cancers, with ER-positive breast cancers the slope is quite gradual. It astonishes me how many late recurrences there can be, but most women in the MA17 trial did very well.
Women with early-stage breast cancer, who are free of recurrence through five years of tamoxifen, have a relatively low risk of recurrence and a good prognosis. In the placebo arm of the trial, the annual risk of a breast cancer event was about two to three percent per year. The absolute benefits of letrozole are relatively modest. In the aggregate, letrozole prevented one breast cancer event per 100 women per year.
In patients with ER-positive disease, the time courses for late recurrences in node-positive and node-negative disease are similar. In MA17, the relative hazard ratio was the same for the benefit of letrozole over placebo in both node-positive and node-negative patients. However, because we presume patients with node-negative disease have a lower residual jeopardy five years out, the absolute benefit of taking letrozole for patients with such a good prognosis is less than if they'd had a lot of positive nodes. Patients with node-positive disease are still at greater residual jeopardy.
Clinical implications of MA17
The risk reduction seen in MA17 included both distant metastases and second breast cancer events - either in-breast recurrence or secondary contralateral breast cancers. These local regional recurrences constituted a relatively large fraction of all the breast cancer events seen in MA17. For most women who have had one breast cancer, their greatest threat to survival is the breast cancer we already know about, rather than a second breast cancer. For the well-informed patient, the data can be interpreted to offer a secondary benefit - chemoprevention.
The use of aromatase inhibitors in prevention is being explored by a number of investigators. The differences in the ATAC trial are relatively modest, but there remains a trend favoring the aromatase inhibitor in terms of preventing second in-breast recurrences or contralateral breast cancers. It suggests there are two things going on - continued control of microscopic distant metastases and ongoing improvement in reducing the risk of primary breast cancer.
Time since completion of tamoxifen and aromatase inhibitor
MA17 was open to women who had finished tamoxifen within the past three months, but we have no data for women who have been off tamoxifen for a longer period. In practice, I consider letrozole therapy for patients who have finished their five years of tamoxifen therapy within the past year. Beyond year six, women who have had no recurrences have an additional period of time during which they've done well, and that means their moving-forward risk is even lower than it was before. It's difficult to know whether or not the data apply to them.
The whole issue of the timing, duration and sequencing of antiestrogen strategies is very interesting, and everyone is looking forward to the results of the Breast International Group/Femara® -Tamoxifen (BIG/FEMTA) study. This large European trial has four arms: (1) five years of an aromatase inhibitor, (2) five years of tamoxifen, (3) two years of tamoxifen followed by three years of an aromatase inhibitor, and (4) two years of an aromatase inhibitor followed by three years of tamoxifen (Figure 1.2).
Aromatase inhibition as intial adjuvant therapy
Either tamoxifen or anastrozole are up-front options for postmenopausal women with ER-positive tumors based on the data from one large randomized trial, the ATAC study. I present that data but also review the decades of experience we've had with tamoxifen. I also review the different side-effect profiles of these medications.
In terms of selection of an aromatase inhibitor up front, there is no data at this point for letrozole and exemestane, therefore, I prefer to go with anastrozole if I'm going to use up-front aromatase inhibition.
Ovarian suppression in the treatment of premenopausal women with breast cancer
The IBCSG is coordinating a series of three nested trials: SOFT, PERCHE and TEXT. These trials address what is probably the most important conceptual question in premenopausal breast cancer right now: Beyond tamoxifen, does planned ovarian suppression benefit patients?
In particular, does it benefit women who receive chemotherapy or who don't receive chemotherapy, and if a woman experiences chemotherapy-related amenorrhea, does she still need ovarian suppression? We will probably not have the data for at least five or 10 years, but these are very important trials that offer a wonderful opportunity for community oncologists to participate in answering this critical question.
Currently, I consider ovarian suppression for two groups of patients. The first group includes patients at high risk - multiple positive nodes, very high-risk tumors - and particularly young women, less than 35 or 40 years of age, who may not go into menopause with chemotherapy. The other group includes women who are at the opposite end of the spectrum - very low-risk tumors, smaller tumors, node-negative - for whom the benefits of chemotherapy are very small. In these women, I present ovarian suppression as an option, not necessarily in addition to chemotherapy but perhaps even instead of it.

Dose-dense adjuvant chemotherapy
The availability of growth factors and better supportive care measures has enabled us to ask very interesting questions about dose schedule and dose intensity. We have to acknowledge the contributions that Larry Norton and his mathematical models have made in this arena.
CALGB-9741 compared the standard three-week schedule of AC followed by paclitaxel to a dose-dense, every-two-week schedule. The study also looked at a question of sequential monotherapy versus concurrent therapy. The analyses suggested that, while there was no clinically important difference between sequential therapy and concurrent therapy, the every-two-week schedule was superior to the every-three-week schedule. If I'm going to give sequential AC and paclitaxel, I give it every two weeks, instead of every three weeks, because of the survival advantage associated with that regimen.
For node-positive patients, I'm most commonly using dose-dense AC followed by paclitaxel. We feel quite comfortable with this regimen.
As a protocol option, we have been exploring dose-dense AC followed by paclitaxel with pegfilgrastim. I would not encourage people to use pegfilgrastim instead of filgrastim outside of a study, although I know it is widely done.
Treatment of patients with metastatic, HER2-positive, ER-positive breast cancer in a nonprotocol setting
In women who have HER2-positive disease and potentially endocrine-sensitive tumors, I start with endocrine therapy for a couple of reasons. First, clearly endocrine therapy can still be effective in HER2-positive breast cancer, and I like to get as much mileage as appropriate from endocrine treatments. There's this myth that these patients don't benefit from endocrine treatment, but that's simply not the case.
Second, the pivotal study from Dennis Slamon, evaluated chemotherapy plus or minus trastuzumab and found that neither estrogen receptor status nor prior endocrine treatment adversely affected response rates or outcomes to combinations of chemotherapy and trastuzumab. I don't believe we burn any bridges by starting with endocrine therapy. When the patient is no longer a candidate for endocrine therapy, I usually introduce chemotherapy. If the tumor is HER2-positive, then I use chemotherapy plus trastuzumab.
Clinical trials of preoperative trastuzumab/chemotherapy in HER2-positive breast cancer
We conducted a pilot program of preoperative trastuzumab and paclitaxel for women with HER2-positive breast cancer. After 12 weeks of preoperative therapy, the patients had surgery and then received four cycles of doxorubicin and cyclophosphamide. There was a very high response, on the order of 70 to 80 percent, with a pathologic complete response rate of approximately 18 to 20 percent. The treatment seemed feasible in that none of the patients developed symptomatic heart failure or other complications.
We followed that study with a trial of preoperative trastuzumab and vinorelbine. Again, we saw very robust response rates and pathologic complete response rates on the same order of magnitude, and subsequent anthracycline-based therapy was found to be feasible following the initial trastuzumab and chemotherapy combination. Preoperative trastuzumab is a fascinating model for exploring how this drug actually works.
We are putting together another pilot combining trastuzumab with one of the platinums and a taxane. While this is not the standard of care for women presenting with Stage III or locally-advanced, HER2-positive disease, we are increasingly considering it as a realistic treatment option for these patients.
Emerging data from the cooperative group trials evaluating changes in LVEF with standard chemotherapy and, in some instances, with trastuzumab-based therapy in the early-stage setting collectively indicate that it's going to be feasible. This does not mean we should be giving trastuzumab to all patients, and we do not do so outside of a clinical trial, but looking ahead a couple of years, I believe we're going to find ways to sequence trastuzumab into adjuvant chemotherapy without prohibitive cardiac toxicity.
First-line therapy for anthracycline-naïve, HER2-positive, metastatic disease
If a woman has a hormone receptor-negative tumor, the only strategy we have is chemotherapy, but if the tumor is HER2-positive, then I give chemotherapy with trastuzumab. Oncologists who prefer to begin with an anthracycline-based regimen as first-line therapy for an HER2-positive tumor presumably do so because of a historic belief that everyone needs an anthracycline up front. I don't believe that's true. Mary Costanza conducted a study for the CALGB at the University of Massachusetts that compared a first-line, anthracycline-based regimen to a nested series of Phase II agents and showed no real difference in survival.
In addition, George Sledge's ECOG trial, probably the best data we have, compared doxorubicin to paclitaxel versus the combination and found no substantial difference in the duration of response or in overall survival for any of those three strategies. We now have a variety of active nonanthracycline-based drugs, and trastuzumab has clearly been shown to improve survival. I think that's the priority, and we should rely on that data rather than falling back on data from the 1970s.
Combination trastuzumab/chemotherapy in the metastatic setting
Several published trials showed the response rate to single-agent trastuzumab is on the order of 30 to 35 percent in patients whose tumors are HER2 3+ by IHC or FISH-positive, so monotherapy is a viable option. However, the response rates to chemotherapy plus trastuzumab are typically twice that, so I usually start with a combination. We've been interested in nontoxic chemotherapy regimens and have done a lot of work with vinorelbine and trastuzumab (Figure 1.3). That combination tends to be well-tolerated, doesn't cause alopecia or nausea, and I find it appealing for patients who don't want more aggressive chemotherapy.
I usually treat with the combination to a point of optimal response and then discontinue the chemotherapy, leaving the patient on trastuzumab. There's no data telling us whether that's good or not, but it spares patients the side effects of chemotherapy, and many women experience extended periods of disease control.
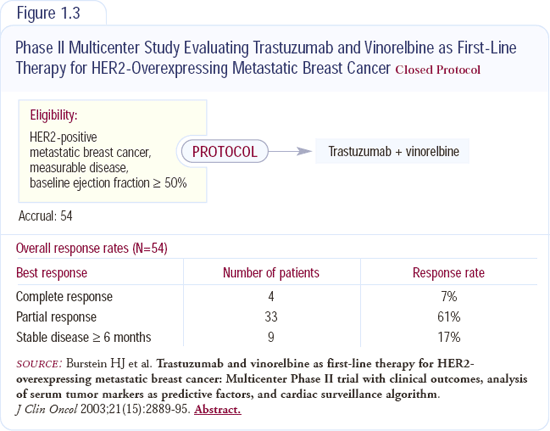
Discontinuing both agents is another viable option, but the patients generally feel they've gotten significant clinical benefit from trastuzumab and, because it's relatively nontoxic and can be offered on an every-three-week treatment schedule, they prefer to continue taking it. Most of my dosing is built around weekly trastuzumab, but if the patient transitions to trastuzumab monotherapy or if we're using an every-three-week chemotherapy cocktail, then I use the every-three-week dosing.
Continuation of trastuzumab beyond disease progression
In patients who experience long periods of disease control with trastuzumab monotherapy and then progress again, restarting them on the original chemotherapy with trastuzumab is very reasonable. If the cancer recurs within a short window, then it's probably time to move on to another chemotherapy agent. I tend to continue the trastuzumab, unless the patient needs an anthracycline or is going on to a protocol that precludes trastuzumab.
This is an area in which we have no data to direct us, so MD Anderson tried to mount a study in which women who progressed on trastuzumab with a taxane would be randomly assigned to vinorelbine with or without trastuzumab. It was a reasonably well-designed study, but there was one major scientific flaw - trastuzumab has a very long half-life, probably three weeks, so there could still be circulating levels of trastuzumab for the first eight to 12 weeks of no trastuzumab treatment, which confounds the endpoint.
The second and more practical problem was that patients were not willing to be randomized - they all wanted to continue taking trastuzumab. That study was closed, but they have tried to reintroduce it through the SWOG. I hope that's successful because it's a very important study.
Oral chemotherapy agents in the treatment of metastatic breast cancer
We continue to be interested in oral chemotherapies in the metastatic setting. They are relatively underexploited in terms of patient convenience and global utilization. Patients like the convenience of oral medicines as long as they don't compromise efficacy, and most of the planet does not have access to sterile IV preparation. We have more and more oral drugs that are efficacious, and it would be marvelous if we could develop effective oral chemotherapy cocktails that could be used in the adjuvant setting.
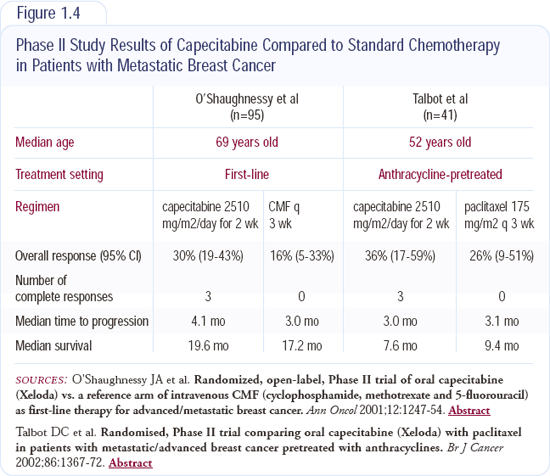
Capecitabine is a very useful and widely used drug that's FDA-approved for anthracycline- and taxane-pretreated breast cancer, but I believe it's a very reasonable first-line agent. Randomized studies comparing capecitabine to traditional CMF and to paclitaxel as first-line treatment have shown generally equivalent results (Figure 1.4). We are currently conducting a Phase I study evaluating the combination of capecitabine and oral vinorelbine. One of the lessons we've learned is that oral chemotherapy is still chemotherapy; we don't obviate side effects like neutropenia just because it's a pill.
Select publications
|
| Dr Burstein is an Assistant Professor of Medicine of the Harvard Medical School Breast Oncology Center at the Dana-Farber Cancer Institute in Boston, Massachusetts. |
|
