| You
are here: Home: BCU 6|2001: Section 1
Section 1
Hormone Replacement Therapy in Breast Cancer Survivors
Editor’s Note: When hormone replacement is utilized
in a postmenopausal woman with an intact uterus, a combination estrogen-progestin
preparation is standard. However, in the past several years, the
impact of progestin use on breast cancer risk has become controversial.
At the 3rd Annual Lynn Sage Breast Cancer Symposium (October 2001),
Malcolm Pike, PhD, of the University of Southern California, opined
that the progestin component of combined therapy represents the
most preventable cause of breast cancer among women in the United
States today, and he estimated that HRT was responsible for causing
approximately 10 percent of breast cancers occurring in postmenopausal
women.
Below are select, edited excerpts of Dr Pike’s presentation
as well as comments from an interview with him shortly after his
presentation. Dr Pike summarizes key evidence suggesting that progestins
substantially contribute to an increased risk for breast cancer.
He also provides alternative strategies to reduce progestin exposure
in HRT and addresses other clinical issues commonly raised in medical
oncology practice.
ESTROGEN VS ESTROGEN-PROGESTERONE REPLACEMENT THERAPY AND BREAST
CANCER RISK
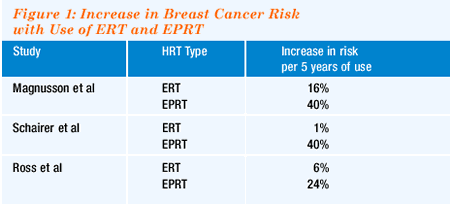
Estrogen-progesterone replacement therapy is the most preventable
cause of breast cancer that’s around today. There have been
three recent large studies 1-3, all of which showed a much greater
increase in breast cancer risk from estrogen-progesterone replacement
therapy than from estrogen replacement therapy alone — maybe
three or four times greater (Figure 1).
Dr Hofseth and her colleagues showed that breast cell mitotic rate
on continuous combined estrogen-progesterone replacement therapy
was more than double that of women on estrogen replacement therapy
alone 4. So, here was the first bit of real nonepidemiological
evidence that this was true. The second set of evidence came from
a randomized trial — the PEPI trial — where Dr Greendale
and her colleagues 5 showed that there was a much greater increase
in mammographic densities of women on estrogen-progestin than on
estrogen alone (Figure 2).
The Overview 6 that was done a few years ago — which reported
on all of the epidemiological studies ever done — showed that
ERT alone increased breast cancer risk about 2% per year of use.
There are arguments about how much longer that increased risk will
last after HRT is stopped, and that question remains unanswered.
A woman will experience a small increased risk of breast cancer,
but if she uses it for just a few years to control hot flashes,
then that would be a completely reasonable thing for her to do —
hot flashes are pretty awful.
—Malcolm Pike, PhD

Select Publications
APPROACHES TO REDUCING BREAST CANCER PROGESTIN EXPOSURE WITH
HRT
One way to manage breast cancer versus endometrial cancer risk
is to give progestins less frequently. Bruce Ettinger 10
in Oakland and Dan Williams 11 at UCLA both showed that if you gave
progestins for 12 to 14 days every three months — instead of
every month — you got complete control of endometrial hyperplasia.
And we showed in an epidemiological study that this was extremely
likely to be successful in preventing endometrial cancer, which
is the primary reason to give a progestin.
Another option is to use an intrauterine device. 12 Progestasert®
(intrauterine progesterone contraceptive system) has to be removed
every 18 months but contains a very low dose of progesterone that
does not get into the circulation. The newer IUD containing progestins
— Mirena ® (levonorgestrel-releasing intrauterine system)
— should last for 10 years. Progesterone can also be delivered
intravaginally, which can change the ratio of concentrations in
the endometrium relative to the blood by a factor of 50.
—Malcolm Pike, PhD

Select Publications
EVALUATION OF CURRENTLY AVAILABLE HORMONE REPLACEMENT
DRUG THERAPIES
Ortho-PREFEST® (estradiol/norgestimate) has just been approved
by the FDA. It’s one milligram estradiol and 0.09 milligrams
of norgestimate — one of the new progestins, and it is given
for three days every six days. This agent is intended to keep estradiol
and progesterone at the highest levels, which is potentially a disaster.
There’s no requirement of the FDA at the current time to show
that new forms of estrogen-progesterone replacement therapy do not
increase breast cancer risk. We must insist on that, because this
is a major cause of breast cancer in the United States at the moment.
HRT IN WOMEN RECEIVING TAMOXIFEN
The premenopausal woman taking tamoxifen has high levels of circulating
estrogen, because tamoxifen is acting as a stimulant to the ovary.
And yet tamoxifen still works. In the P-1 study 14 and in other
randomized trials, tamoxifen was very effective in preventing breast
cancer as well as contralateral disease in premenopausal women.
So, for a postmenopausal woman on an antiestrogen, a small dose
of estrogen probably doesn't matter.
—Malcolm Pike, PhD
Select Publications

IBIS II TRIAL OF ANASTROZOLE FOR BREAST CANCER
PREVENTION
These trials must take the same sort of approach that we used with
SERMs — to look at all the effects including bone and lipids.
There’s no real question that if you block all estrogen, you’ll
get a tremendous effect on breast cancer risk. Will that effect
be as great as tamoxifen? I don’t know, because the effect
of tamoxifen is really quite astounding — a 50% reduction in
risk, effective instantaneously.
You might get that with an aromatase inhibitor but we don’t
know that. In normal women, breast cancer rates increase sharply
in premenopausal women and much less after menopause. But between
premenopause and postmenopause — in the perimenopausal period
— breast cancer rates actually drop temporarily. And that must
be because the cells were living in this estrogen-progesterone environment,
and now they’re changing to a low-level estrogen environment.
Also, in premenopausal women, the drop in breast cancer rates with
an oophorectomy is as sharp as with tamoxifen. So, any real drastic
change in hormones will do this.
With anastrozole, it will depend on how large the change is and
where you think the dose response curve is. A postmenopausal woman
has 20 picograms per ml of estradiol, and with anastrozole s h e
’s going to have nothing. But you’re also going to have
to look at her bones and lots of other things. It’s the same
problem as with SERMs. You’re going to have to prove that all
these other things are okay, not just that you’re reducing
breast cancer risk, because I’m sure you will.
—Malcolm Pike, PhD
Select Publications
|
