| You
are here: Home: BCU 4|2001: Section1
Section 1
Breast
Cancer in the elderly
INTERGROUP
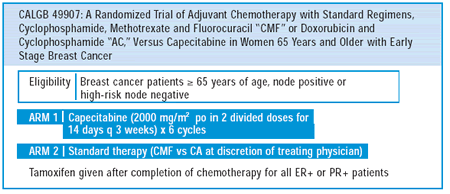
TRIAL OF ADJUVANT CHEMOTHERAPY IN OLDER WOMEN
We
are currently close to launching a trial for women age 65 and older
who have either node-positive or high-risk, node-negative breast
cancer. Patients will be randomized to either capecitabine or standard
therapy with either CMF or CA. This will be an equivalence trial
to see if oral capecitabine for six courses is equivalent to either
CMF or CA. Quality of life and the influence of co-morbidity on
outcome will also be studied, as will the functional status of the
patients. We’re very excited about this, because we believe
that if capecitabine is equivalent to more intensive regimens, it
might be very attractive for many patients as an adjuvant regimen.
I believe that a lot of physicians will be willing to put patients
on this trial. The patients are there, and I feel confident we will
meet accrual.
If you look
at Phase II trials in metastatic breast cancer as second-and third-line
therapies, there is now a reasonable database for capecitabine demonstrating
response rates of about 20 to 30 percent, 4-7 which really
is comparable to taxanes, vinorelbine and other very active agents.
So, if you look at capecitabine as a single agent, it fits in. Taxanes
have been extensively compared to regimens like CAF and CMF and
have proven to be as good, if not superior, and so if you take a
Boolean approach, capecitabine should be reasonable to consider
for an equivalence trial to CA or CMF.
There is also
a very small comparison of capecitabine versus CMF in metastatic
disease where the response rate was higher, although not significantly
for capecitabine. 8 Certainly it did not look detrimental.
I think that
the package insert dose of 2,500 mg/m 2 per day of capecitabine
is too high, and in our adjuvant trial, we’re going to start
at 2,000 mg, and we’re even going to watch that very closely.
I suspect that we’re not going to lose much by lowering the
dose, because lowering the dose will still leave you with very high-quality
serum levels that should be effective.
The major toxicity
we expect with capecitabine is hand-foot syndrome, diarrhea and
occasionally stomatitis,which will be monitored very closely. I’m
hoping that it won ’t be that bad an experience at all,and
logistically, it should be much more user-friendly for patients.
—Hyman
Muss, MD
-
Review Select Publications
-
Other Clinical Trials
Page
3 of 3
Back
|
