|
You
are here: Home: BCU 4|2001: Section1
Section 1
Breast
Cancer in the elderly
CLINICAL
TRIALS IN ELDERLY BREAST CANCER PATIENTS
Many of our
earlier clinical trials had age cutoffs — for example at age
70. Now 70-year-old women play tennis. We did a study 2 in the CALGB
that addressed the issue of protocol accrual in elderly patients
in which we matched older and younger women who were eligible for
a breast cancer clinical trial at the same institution, who had
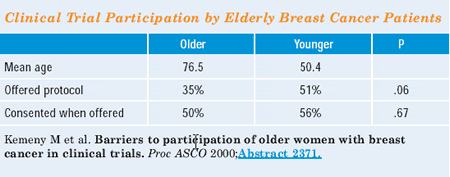
the same physician. We found that women 65 and older were offered
a trial 35 percent of the time, but women who were less than 65
were offered a trial 51 percent of the time. What was interesting
was that of the patients who were offered trial participation, about
half in each group agreed. So, if you offered an older woman a trial,
she went on with the same frequency.
Discussions
about protocol design must consider issues such as co-morbidity
and co-existing illness, which are certainly age-related. And how
should that be factored in? A wonderful example of looking at co-existing
illness was the superb analysis of Mitch Gail 3 and his colleagues
looking at the potential benefits of tamoxifen prevention factored
in with United States data concerning stroke, myocardial infarction
and endometrial cancer. These are data we really don’t have
available at all in breast cancer adjuvant chemotherapy in older
women. Perhaps diseases that we believe shorten lives and lower
quality of life are not that bad, and women may be willing to enter
adjuvant chemotherapy trials to extend their lives, even though
their general medical health may not be that good.
—Hyman
Muss, MD

Page
2 of 3
Back | Next
|
|
