
 |
||||||||

The optimists among us would like to think that May 16, 2005 was a turning point in oncology research. With George Sledge at the helm of the now legendary ASCO “education session,” we all got one hell of an education about the potential of targeted cancer therapy.
George’s partner in progress, Kathy Miller, set the tone for this highly memorable afternoon by presenting the first report of successful anti-angiogenic therapy for breast cancer, and the day got better and better as a series of spectacular adjuvant trastuzumab data explosions followed from the NSABP, NCCTG and BIG cooperative groups.
In an instant on that afternoon in Orlando, one of the major questions in breast cancer clinical research was definitively addressed by the impressive results from three well-designed and appropriately powered randomized adjuvant trials. This remarkable event effectively validated the molecular targeted approach to common cancers and substantiated our hope and optimism that this might be the end of the beginning of a successful war on cancer.
Now there are exciting new adjuvant studies in HER2-positive breast cancer being developed, generally looking to add another biologic like bevacizumab or lapatinib to the recipe and to individualize treatment based on tumor markers such as TOPO II and cMYC. These studies join a panoply of other interesting and worthwhile ongoing clinical investigations in all stages of the disease, and one can cautiously conclude that we have entered a new and exciting era of breast cancer clinical research.
The only problem is that we’re still in the dark ages in terms of accruing patients onto trials, and we need to do more than just bitch and moan about it. To contribute to the solution of this critical situation, our CME group, Research To Practice, is again partnering with Dr Kent Osborne, co-director of the San Antonio Breast Cancer Symposium, on a clinical trials education initiative that began in 2001.
This information platform/pep rally focuses on the current generation of ongoing studies and new trial concepts under development. Our goal is to encourage oncologists and other oncology healthcare professionals to increase their already staunch efforts in clinical research and to make a renewed commitment to investigate, participate and educate as follows.
INVESTIGATE
The enclosed breast cancer clinical trials audio program and this print CME guide summarize some of the most important and innovative current breast cancer clinical trials, and provide additional insights and perspectives into their relevance and significance.

By creating this broad overview, our goal is to
provide a concise and easy-to-use method to
learn about current clinical trials and to understand
not only the research relevance but also
the exciting potential benefits to participating
patients.
Many common clinical situations in breast cancer have suboptimal available therapeutic options, and after witnessing the trastuzumab miracle, we now clearly understand that studies such as the upcoming Intergroup adjuvant bevacizumab trial (see page 68), as discussed by Dr George Sledge on the audio program, can provide patients with the opportunity to receive promising, relatively nontoxic and otherwise unavailable therapies. Many other current trials offer potential benefits to participating patients, including SWOG-S0307 (page 30), discussed on the audio program by principal investigator Dr Julie Gralow. This study of three different bisphosphonates in the adjuvant setting has some of the broadest eligibility criteria of any current major breast cancer trial.
The study accepts essentially any patient with invasive disease at high enough risk to receive some form of adjuvant systemic therapy, including endocrine therapy alone. SWOG-S0307 is asking an important question, and there should be absolutely no reason why we can’t quickly recruit 6,000 patients to find an answer.
Another exciting aspect of the new generation of breast cancer clinical trials is that tissue correlative studies have now become virtually standard, and studies such as ACOSOG-Z1031 (page 14), which is being led by Dr Matt Ellis, hold the promise of unlocking one of the oldest questions in breast oncology: Why do some patients with ER-positive tumors not respond to endocrine therapy? This simple but spectacular and much needed study is comparing the three available aromatase inhibitors — anastrozole, letrozole and exemestane — as neoadjuvant therapy for postmenopausal women with ER-positive tumors.
After years of Drs Mike Dixon and John Robertson telling us about their fascinating neoadjuvant endocrine trials across the Pond, we now have a highly noteworthy North American preop endocrine trial, and this study is just a prelude to a planned follow-up protocol that will compare neoadjuvant chemotherapy to the best AI (or dealer’s choice if no differences show up in the current study). Vegas is currently booking the AIs over chemo with three points.
The new generation of trials is also asking a number of questions with enormous practical implications in terms of safety and quality of life. For example, after new data sets were presented at ASCO 2006 on cardiac toxicity associated with adjuvant anthracyclines, clinicians are now thinking twice about even four cycles of AC for patients with cardiovascular risk factors such as hypertension.
Help could be on the way in the form of several ongoing adjuvant trials investigating nonanthracycline regimens. My favorite is CALGB-40101, which compares dose-dense paclitaxel to dose-dense AC. Oncologists and cardiologists are keeping their fingers crossed that four cycles of paclitaxel with growth factors might provide adequate efficacy with a better safety profile for patients at lower risk.
To assist San Antonio attendees in keeping clinical research top of mind, another facet to this education initiative is our “protocol water bottles,” which will be distributed at the Clinical Trials education booth in Hall C. While staving off dehydration during the long daily sessions, imbibers are encouraged to give some thought to the available study designs.
PARTICIPATE
With the hope that increasing exposure and discussion of clinical trials might be helpful, and to spice things up a bit, we are proud to announce the first annual “Design A Breast Cancer Clinical Trial Challenge.” This unique contest — dubbed by Hal Burstein as the “American Idol” approach to protocol design — is rather simple in concept: All you need to do to enter is propose a theoretical protocol that you believe would be worthwhile to implement.
An elite panel of judges will deliberate the merits of each entry and will select five winners. The authors of the victorious trial designs will be invited to participate in a special audio-recording roundtable and stone crab feast hosted in balmy Miami in February 2007. The edited audio proceedings from that discussion will be presented to our national audience as part of the Breast Cancer Update audio series.
We are also launching a new web-based interface to support this effort — DesignATrial.com. The site will allow for an ongoing, international interchange on new clinical trial ideas.
We ask participants in “Design A Trial” to stretch their imaginations and put funding practicalities aside. You have been given a monstrous grant to do your trial the way it needs to be done. Tell us all about it.
FIRST ANNUAL DESIGN A CLINICAL TRIAL CHALLENGE
Submit your innovative ideas for future breast cancer clinical trials to DesignATrial.com or visit our clinical trials education booth in Hall C at San Antonio.
Tell us briefly what you want to study and why. Our panel of esteemed clinical researchers will evaluate all the entries and vote on the best. The authors of the five most interesting trial designs as determined by our panel will be invited to present and discuss their ideas during a special educational roundtable and audio recording session in Miami, Florida in February 2007.
All entries must be received by December 31, 2006 and winners will be notified by January 22, 2007. So go ahead, think outside of the box — then think outside of that box and let us know what you come up with. Here’s my entry:
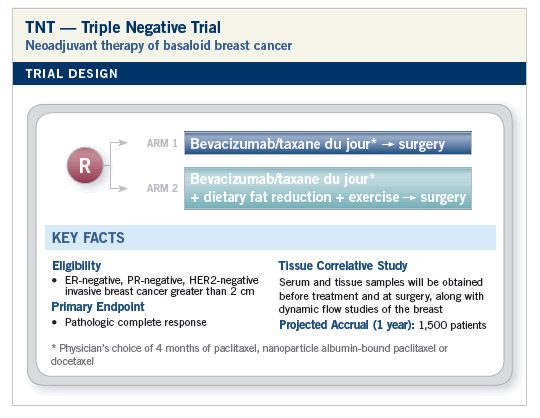
RATIONALE
Seemed like a good idea at the time. No, seriously, the WINS study cannot be ignored. Why did women with ER-negative tumors have fewer relapses with dietary fat reduction? Subset mischievousness? I don’t think so.
Also, how does one explain the repeated signals about exercise and relapse in breast and colon cancer? Is it all about insulin, or is it more complicated than that?
Whatever the mysterious effects of these interventions are, would there be synergy with maybe our best known systemic therapy for triple-negative tumors?
My personal choice for a taxane would be nab as opposed to the other available taxanes. I don’t want premedications messing up my perioperative cocktail, and steroids might make it difficult for patients to control their food intake. Postoperative therapy, if any, will be the physician’s choice. All patients will also be eligible to receive postop exercise and nutritional interventions. Let me know what you think of this design and submit your ideas at DesignATrial.com. Maybe I’ll try to present my TNT trial at the NCI meeting on preoperative therapy next March (http://ctep.cancer.gov/bcmeeting).
| One Patient’s Perspective on “Design A Trial” |
| Editor: As this program was about to go to print, I received the following email from Mrs Sandra Martinez, who for the last nine years has been the lead transcriptionist for our CME group. In 2004, Mrs Martinez was diagnosed with breast cancer, an experience that further increased her already intense interest in oncology.
Life for a cancer patient isn’t just about keeping breathing, it’s also about enjoying every breath you take. Depression — usually present whether recognized or not — plays a huge role in this process. I also wonder about doing a trial that evaluates laughter therapy. No, I don’t know how to accomplish all this; I just know what’s important to me as a patient and what has helped me cope with some of the many challenges a cancer patient faces. Just my thought for the day. — Sandra
|
EDUCATE
If you are already a believer in the importance of clinical trials, we want to make it easier for you to spread the word, and to do this we have produced a PowerPoint slide presentation and Speaker’s Kit based on the content of this monograph, which is available at our education exhibit at the conference and online at BreastCancerUpdate.com/ClinicalTrials. We encourage you to take home a complimentary copy of this program and incorporate the slides into your lectures and presentations. The May 16th message is out there. We have the technology. Let’s get this thing done.
— Neil Love, MD
NLove@ResearchToPractice.net
Special thanks to SABCS co-director Dr Kent Osborne for working with us to develop this initiative and for reviewing the enclosed education materials.
Editor’s Note:
Let’s get this thing done.
Prologue:
The adjuvant trastuzumab clinical research to
practice model
- Select publications
Breast Cancer Clinical Trials:
Neoadjuvant Therapy