|
 Home:
Educational Supplement: Appendix
Home:
Educational Supplement: Appendix
Who
Should Not Get Tamoxifen?
C.
Kent Osborne, M.D.
Tamoxifen is
a selective estrogen receptor modulator (SERM) that demonstrates
antiestrogenic effects in breast cancer cells and in vaginal mucosa,
and estrogenic effects in bone, endometrium, and the liver. These
dual effects suggest that women taking tamoxifen might benefit from
the drug’s inhibitory effects on the cancer as well as from
its agonist effects to maintain bone density and to lower cholesterol.
Adverse effects would be expected from the estrogen-like stimulation
of the endometrium.
Clinical trials
of tamoxifen were started more than 25 years ago. The drug has an
established role in the treatment of estrogen receptor (ER)-positive
metastatic breast cancer and in adjuvant therapy in patients with
primary breast cancer, and an evolving role in treating ductal carcinoma
in situ (DCIS) and in prevention of breast cancer.
Invasive
Breast Cancer and Tamoxifen
Individual randomized
clinical trials as well as meta-analysis of all adjuvant trials
clearly demonstrate that 5 years of adjuvant tamoxifen reduces the
risk of recurrence and reduces mortality of women with ER-positive
early breast cancer (see table 1).
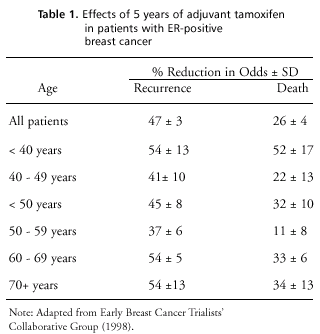
Reductions in
recurrence and mortality are seen irrespective of age, with patients
under 40 years of age benefiting as much as older patients so long
as the tumor expresses ER. Overall, such patients have a 40 to 50
percent annual reduction in the odds of recurrence. Patients whose
tumors are strongly ER-positive have a 60 percent proportional reduction
in recurrence. In addition, there is a 50 percent reduction in the
incidence of contralateral breast cancer in patients treated with
adjuvant tamoxifen.
Despite the
effects of tamoxifen on cholesterol, no reduction in cardiac mortality
has been observed in meta-analysis of tamoxifen adjuvant trials.
An increased incidence of well-differentiated endometrial cancer
as a result of tamoxifen therapy has been documented, although the
beneficial effects of tamoxifen on breast cancer far outweigh that
adverse event. An important question is whether patients with ER-negative
tumors receive some benefit with tamoxifen adjuvant therapy, either
in terms of reducing the risk of recurrence and death from their
primary tumor or in terms of the ancillary benefits in relation
to contralateral breast cancer, cholesterol, and bone density. The
meta-analysis shows no recurrence or mortality benefit in patients
whose tumors are ER-negative in either the presence or absence of
chemotherapy (EBCTCG, 1998). Two recently reported but as yet unpublished
adjuvant trials were prospectively designed to determine the benefits
of tamoxifen in ER-negative patients. Intergroup trial 0102 randomized
high-risk node-negative patients, both ER-positive and ER-negative,
to chemotherapy alone (CMF or CAF) or chemotherapy plus 5 years
of tamoxifen (Hutchins, Green, Ravdin, et al., 1998). That trial
showed no overall benefit with tamoxifen in the ER-negative subset,
with a trend toward an unfavorable outcome in the premenopausal
group. Patients with ER-positive tumors received significant benefit.
The other study,
NSABP B23, randomized ER-negative patients to chemotherapy alone
or chemotherapy plus tamoxifen (Fisher, Anderson, Wolmark, et al.,
2000). That trial also showed no benefit from the addition of tamoxifen.
Thus, neither
the meta-analysis of all trials nor the two large randomized clinical
trials prospectively designed to answer the question show a recurrence
or survival benefit for adjuvant tamoxifen in the ER-negative subset.
Nonetheless, many physicians prescribe tamoxifen for ER-negative
patients because of its potential benefit in reducing contralateral
breast cancer. Emerging data that will be presented at this conference,
however, suggest that, in marked contrast to the R-positive subset,
the incidence of contralateral breast cancer is not reduced by tamoxifen
in patients with ER-negative primary tumors. Thus, on the basis
of current data, there is no justification for the use of adjuvant
tamoxifen in patients whose tumors are ER-negative.
Ductal Carcinoma
In Situ (DCIS)
Trials are in
progress evaluating the role of tamoxifen in patients with DCIS
following mastectomy or breast conservation surgery. Trials on invasive
breast cancer suggest that tamoxifen may reduce the risk of ipsalateral
breast cancer recurrence in patients treated with breast preservation
as well as reduce the incidence of contralateral breast cancer.
Such patients have little risk of dying of distant recurrence from
their initial breast cancer, and the use of tamoxifen in these patients
might be viewed as chemoprevention, much like its use in patients
with increased breast cancer risk. One large randomized trial (NSABP
B-24) has been completed, and early results have been published
(Fisher, Dignam, Wolmark, et al., 2000). More than 1,800 women with
DCIS with either positive or negative surgical margins were randomized
to lumpectomy plus radiation or to lumpectomy plus radiation plus
tamoxifen. Women in the tamoxifen arm had significantly fewer invasive
and noninvasive breast cancers in the ipsalateral breast and also
a reduction in contralateral breast cancer. Slightly more patients
on tamoxifen developed venous thrombotic events, but there have
been no treatment-related deaths. As expected, there was an increased
rate of endometrial cancer in patients treated with tamoxifen. These
data suggest that tamoxifen treatment can be considered in women
treated with lumpectomy and radiation therapy to prevent both ipsalateral
and contralateral invasive breast cancer. Tamoxifen would also be
expected to reduce the incidence of contralateral breast cancer
in patients treated by mastectomy, but the drug has not been studied
definitively in this group. The role of ER status in predicting
tamoxifen benefit in patients with DCIS has not been examined.
Prevention
Three randomized
trials have addressed the role of tamoxifen in breast cancer prevention.
One of these, the P-1 trial run by the NSABP and reported by Fisher
and colleagues (Fisher, Costantino, Wickerham, et al., 1998) was
a large double-blind randomized trial involving more than 13,000
women with an increased breast cancer risk receiving either placebo
or tamoxifen for 5 years. That trial showed a 50 percent reduction
in the incidence of breast cancer after 3 to 4 years of followup.
There was also a reduction in bone fractures, but no change in cardiac
mortality. The incidence of endometrial cancer increased, as expected,
but thus far there are no deaths from endometrial cancer in patients
in the tamoxifen arm. A slight increase in the need for cataract
surgery in women with preexisting cataracts and a slight increase
in thromboembolic events were also reported. Similar data were observed
in a trial using raloxifene for osteoporosis (Cummings, Eckert,
Krueger, et al., 1999). However, the reduction in breast cancer
incidence observed in the latter trial is considered less definitive,
since it was a secondary endpoint. Nevertheless, data from that
trial add weight to the evidence that SERM therapy reduces the incidence
of breast cancer in women who do not yet manifest the disease.
Two other trials
using tamoxifen in prevention have been reported (Powles, Eeles,
Ashley, et al., 1998; Veronesi, Maisonneuve, Costa, et al., 1998).
The trial by Powles and colleagues was a feasibility study randomizing
more than 2,000 women who had an increased risk of breast cancer
based on family history. Overall, the trial showed no significant
reduction in breast cancer incidence, although there was a reduction
in the number of ER-positive breast cancers. Interpretation of the
evidence from that trial, however, suffers because of the concomitant
use of estrogen replacement therapy in many of the women and by
its smaller size and initial design as a pilot feasibility trial.
The study by Veronesi and colleagues involved 5,000 women who had
previously undergone hysterectomy. It showed no overall benefit
for tamoxifen, although there was a trend toward reduction in ER-positive
breast cancers. Interpretation of the data from that trial is also
clouded by the use of hormone replacement therapy by some patients
and from a high patient dropout rate.
The cumulative
evidence at this time suggests that in high-risk women, 5 years
of tamoxifen reduces the incidence of in situ and invasive breast
cancer in both pre- and postmenopausal women. A risk-benefit analysis
suggests that greater relative benefit will be observed in younger
women (Gail, Costantine, Bryant, et al., 1999). Although many questions
remain to be answered, tamoxifen can be considered for women meeting
the criteria of eligibility for the P-1 trial (Chlebowski, Collyar,
Somerfield, et al., 1999). Women not meeting those criteria should
not receive tamoxifen until the data clearly demonstrate a favorable
risk-benefit ratio.
Conclusion
and Future Direction
There are no
data to support the use of tamoxifen in patients with ER-negative
invasive breast cancer. Tamoxifen can be considered in certain subsets
of women with DCIS and in women at increased risk for breast cancer.
Women with an average risk for developing breast cancer should not
receive tamoxifen at this time. Future studies should address the
optimal duration of tamoxifen for treatment and prevention, the
value of ER expression in predicting the tamoxifen benefit for patients
with ductal carcinoma in situ, the risk-benefit profile obtained
by longer followup of patients in prevention trials, health economic
outcomes, the role of tamoxifen in tumor prevention in women with
hereditary susceptibility to cancer, and trials of other SERMs with
a more favorable therapeutic ratio.
References
Chlebowski RT,
Collyar DE, Somerfield MR, Pfister DG. American Society of Clinical
Oncology technology assessment on breast cancer risk reduction strategies:
tamoxifen and raloxifene. J Clin Oncol 1999;17:1939-55. Abstract.
Cummings SR,
Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The
effect of raloxifene on risk of breast cancer in postmenopausal
women: results from the MORE randomized trial. JAMA 1999;281:2189-97.
Abstract.
Early Breast
Cancer Trialists’ Collaborative Group (EBCTCG). Tamoxifen for
early breast cancer: an overview of the randomised trials. Lancet
1998;351:1451-67. Abstract.
Fisher B, Anderson
S, Wolmark N, Tan-Chiu E. Chemotherapy with or without tamoxifen
for patients with ER-negative breast cancer and negative nodes:
results from NSABP B23. [abstract]. Proc Am Soc Clin Oncol 2000;19:72a.
Abstract.
Fisher B, Costantino
JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen
for the prevention of breast cancer: report of the National Surgical
Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst
1998;90:1371. Abstract.
Fisher B, Dignam
J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen
in treatment of intraductal breast cancer: National Surgical Adjuvant
Breast and Bowel Project B-24 randomised controlled trial. Lancet
1999;353:1993-2000. Abstract.
Gail MH, Costantino
JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, et al. Weighing
the risks and benefits of tamoxifen treatment for preventing breast
cancer. J Natl Cancer Inst 1999;91:1829-46. Abstract.
Hutchins L,
Green S, Ravdin P, et al. CMF versus CAF with and without tamoxifen
in high-risk node-negative breast cancer patients and a natural
history follow-up study in low-risk node-negative patients: first
results of intergroup trial INT 0102. [abstract]. Proc Am Soc Clin
Oncol 1998;17:1a. Abstract.
Powles TJ, Eeles
R, Ashley S, Easton D, Chang J, Dowsett M, et al. Interim analysis
of the incidence of breast cancer in the Royal Marsden Hospital
tamoxifen randomised chemoprevention trial. Lancet 1998;352:98-101.
Abstract.
Veronesi U,
Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C, et al.
Prevention of breast cancer with tamoxifen: preliminary findings
from the Italian randomised trial among hysterectomised women. Lancet
1998;352:93-7. Abstract.
|
|

