|
 Home:
Educational Supplement: Appendix
Home:
Educational Supplement: Appendix
Side
Effects of Chemotherapy and
Combined Chemohormonal Therapy
Eric
P. Winer, M.D.
The decision
to receive chemotherapy (or concurrent chemohormonal therapy) involves
careful consideration of both the potential benefits of therapy
and the possible risks. The side effects associated with chemotherapy
to treat breast cancer are substantial, and include some common
short-term effects (usually present during the period of treatment)
as well as the possibility of late toxicity. These side effects
vary, depending on the specific agents used in the adjuvant regimen
as well as the size of the dose and the duration of treatment. There
is also considerable variability across individuals, but our ability
to predict which patients will have more severe toxicity is very
limited. This review will focus on the short- and long-term toxicity
seen with the most commonly used adjuvant chemotherapy (and chemohormonal)
regimens, including CMF, CAF, CEF, AC, and AC followed by T [C=cyclophosphamide,
M=methotrexate, F=fluorouracil, A=doxorubicin, E=epirubicin, and
T=paclitaxel].
Short-Term
Side Effects
For the purposes
of this review, the short-term side effects of chemotherapy occur
during the course of treatment and resolve (in some cases gradually)
with the completion of therapy. The most common forms of toxicity
include emesis (nausea and vomiting), neutropenia with risk of febrile
neutropenia, alopecia, mucositis, neuropathy, and fatigue (Fisher,
Anderson, Wickerham, et al., 1997; Levine, Bramwell, Pritchard,
et al., 1998; Henderson, Berry, Demetri, et al., 1998). With the
exception of neuropathy, most side effects resolve promptly upon
the completion of treatment. In recent years, fatigue has been recognized
as an important toxicity of chemotherapy. Mild to moderate fatigue
is frequent with adjuvant chemotherapy, but it has generally not
been reported as part of the toxicity assessment in clinical trials.
For this reason, it is difficult to compare the severity of fatigue
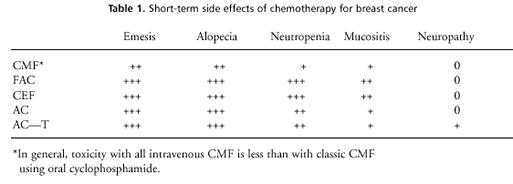
across regimens. Table 1 lists the other side effects rated on a
0, +, ++, and +++ scale, based on the frequency and severity of
the toxicity.
Thromboembolic
complications can also be seen with chemotherapy regimens (Levine,
Gent, Hirsh, et. al., 1988). The risk of thromboembolic events appears
to be increased when chemotherapy and hormonal therapy are administered
concurrently.
Long-Term
Side Effects
For most patients
and clinicians, the long-term side effects of chemotherapy are of
greater concern than the acute short-term effects (Burstein, Winer,
2000). Among the late or chronic effects of chemotherapy, those
of greatest concern include (1) premature menopause; (2) weight
gain; (3) cardiac toxicity from anthracyclines; (4) secondary leukemia;
and (5) cognitive dysfunction. Each of these late effects is considered
separately.
Premature
Menopause
For premenopausal
women with breast cancer, the possibility of experiencing treatment-induced
ovarian failure is a major concern (Burstein, Winer, 2000; Bines,
Oleske, Cobleigh, 1996). It is beyond the scope of this review to
comment on the potential benefits of ovarian failure in relation
to cancer recurrence. There is little question, however, that premature
ovarian failure results in a number of undesired consequences, including
infertility, sexual difficulties, accelerated bone loss, and increased
risk of vascular disease. The risk of ovarian failure with chemotherapy
is related to the age of the woman, the specific chemotherapy agents
used, and the cumulative dose. Cyclophosphamide and doxorubicin
are among the agents most often associated with ovarian failure,
although a treatment course limited to four cycles of AC is more
often associated with preservation of ovarian function than either
6 months of CMF or FAC. Some women will experience periods of ovarian
suppression during the course of treatment and in the months following
therapy, with restoration of ovarian function after a variable time
interval. To what extent a course of chemotherapy may decrease fertility
in women who maintain or resume regular menstrual cycles after treatment
is unknown. Also unknown is whether women who complete chemotherapy
with intact ovarian function will ultimately experience menopause
at a younger age than they would have in the absence of chemotherapy.
Weight Gain
Weight gain
is a well-recognized complication of chemotherapy (Demark-Wahnefried,
Winer, Rimer, 1993). In general, weight gain has been more pronounced
in premenopausal than in postmenopausal women. The mechanism of
weight gain is uncertain, but it does not appear to be a consequence
of excess intake. It is unclear to what extent long-term weight
gain is a problem after a course of chemotherapy. To what extent
weight gain can be modified through behavioral intervention is also
unknown.
Cardiac Dysfunction
The widespread
use of anthracyclines has raised concern about the potential for
cardiac dysfunction following adjuvant therapy. In most doxorubicin-containing
regimens, the cumulative dose of doxorubicin is in the range of
240-360 mg/M 2 . With these cumulative doses, the risk of clinical
congestive heart failure following therapy is thought to be less
than 1 percent (Shan, Lincoff, Young, 1996; Valagussa, Zambetti,
Biasi, et al., 1994). There are rare patients who present with congestive
heart failure with doses in this range. Although higher cumulative
doses of epirubicin are used in epirubicin-containing regimens,
this anthracycline is less cardiotoxic than doxorubicin on a mg
to mg basis. To date, long-term followup of patients treated with
anthracyclines in the adjuvant setting is limited. In a recent report,
more than 300 women who were a median of 10.8 years from diagnosis
underwent cardiac evaluation, including an echocardiogram (Gianni,
Zambetti, Moliterni, 1999). Women who had received anthracyclines
in the adjuvant setting did not have clinical symptoms of cardiac
dysfunction, but a higher proportion of women who had received anthracyclines
compared to those who had CMF alone were reported to have a borderline
or decreased left ventricular ejection fraction.
Secondary
Leukemia
Breast cancer
patients treated with adjuvant chemotherapy have a slightly increased
risk of leukemia. With CMF-type regimens, the increased risk appears
to be negligible (Curtis, Boice, Stoval, et al., 1992; Tallman,
Gray, Bennett, et al., 1995). Anthracycline-based regimens are associated
with a greater risk, though still of relatively small magnitude.
In a series of patients treated with FAC at the M.D. Anderson Cancer
Center, the risk of leukemia at 10 years was estimated to be 1.5
percent. The median time to diagnosis of leukemia was 66 months
(Diamandidou, Buzdar, Smith, et al., 1996). Recent reports from
the NSABP (B-22 and B-25) have also indicated an increased risk
of acute myelogenous leukemia and myelodysplastic syndrome in women
treated with AC (standard dose doxorubicin with standard or dose-intensified
cyclophosphamide) (Fisher, Anderson, Wickerham, et al., 1997; Fisher,
Anderson, DeCillis, et al., 1999).
Cognitive
Dysfunction
Recent reports
have suggested that chemotherapy—both high dose and standard
dose therapy—may be associated with cognitive dysfunction (Brezden,
Phillips, Abdolell, et al., 2000; van Dam, Schagen, Muller, et al.,
1998). The studies have not been definitive, however, and additional
research is needed to determine if cognitive difficulties are a
consequence of adjuvant chemotherapy.
Summary
In summary,
adjuvant chemotherapy (with or without concurrent hormonal therapy
) is associated with a range of acute forms of toxicity which generally
resolve once treatment has been completed. With the exception of
premature menopause, long-term toxicity is generally quite rare.
However, these uncommon late effects need to be considered in making
decisions about adjuvant therapy, particularly when a patient is
at low risk of disease recurrence and the absolute benefit of treatment
is of small magnitude. Further research is needed to characterize
fully the spectrum of late forms of toxicity following adjuvant
chemotherapy.
References
Bines J, Oleske
DM, Cobleigh MA. Ovarian function in premenopausal women treated
with adjuvant chemotherapy for breast cancer. J Clin Oncol 1996;14:1718–29.
Abstract.
Brezden CB,
Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function
in breast cancer patients receiving adjuvant chemotherapy. J Clin
Oncol 2000;18:2695–701. Abstract.
Burstein HJ,
Winer EP. Primary care for survivors of breast cancer. N Eng J Med
2000;343:1086–94. No Abstract Available.
Burstein HJ,
Winer EP. “Reproductive Issues.” In: Harris JR, Lippman
ME, Morrow M, Osborne CK (ed.): Diseases of the Breast, second edition.
New York: Lippincott, 2000, 1051–9. No Abstract Available.
Curtis RE, Boice
JD Jr, Stovall M, Bernstein L, Greenberg RS, Flannery JT, et al.
Risk of leukemia after chemotherapy and radiation treatment for
breast cancer. N Eng J Med 1992;326:1745–51. Abstract.
Demark-Wahnefried
W, Winer EP, Rimer BK. Why women gain weight with adjuvant chemotherapy
for breast cancer. J Clin Oncol 1993;11:1418–29. Abstract.
Diamandidou
E, Buzdar AU, Smith TL, Frye D, Witjaksono M, Hortobagyi GN. Treatment-related
leukemia in breast cancer patients treated with fluorouracil-doxorubicin-cyclophosphamide
combination adjuvant chemotherapy: the University of Texas M.D.
Anderson Cancer Center experience. J Clin Oncol 1996;14:2722-30.
Abstract.
Fisher B, Anderson
S, Wickerham DL, DeCillis A, Dimitrov N, Mamounas E, et al. Increased
intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide
regimen for the treatment of primary breast cancer: findings from
National Surgical Adjuvant Breast and Bowel Project B-22. J Clin
Oncol 1997;15:1858–69. Abstract.
Fisher B, Anderson
S, DeCillis A, Dimitrov N, Atkins JN, Fehrenbacher L, et al. Further
evaluation of intensified and increased total dose of cyclophosphamide
for the treatment of primary breast cancer: findings from National
Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol 1999;17:3374–88.
Abstract.
Gianni L, Zambetti
M, Moliterni A. Cardiac sequelae in operable breast cancer after
CMF + doxorubicin + irradiation. Proc Am Soc Clin Oncol 1999;18:255.
Abstract.
Henderson IC,
Berry D, Demetri G, et al. Improved disease-free and overall survival
from the addition of sequential paclitaxel but not from the escalation
of doxorubicin dose level in the adjuvant chemotherapy of patients
with node-positive breast cancer. Proc Am Soc Clin Oncol 1998;17:390A.
Abstract.
Levine MN, Gent
M, Hirsh J, Arnold A, Goodyear MD, Hryniuk W, et al. The thrombogenic
effect of anticancer drug therapy in women with stage II breast
cancer. N Eng J Med 1988;318:404–7. Abstract.
Levine MN, Bramwell
VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zahra H, et al. Randomized
trial of intensive cyclophosphamide, epirubicin, and fluorouracil
chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil
in premenopausal women with node-positive breast cancer. J Clin
Oncol 1998;16:2651–8. Abstract.
Shan K, Lincoff
AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med
1996;125:47–58. Abstract.
Tallman MS,
Gray R, Bennett JM, Variakojis D, Robert N, Wood WC, et al. Leukemogenic
potential of adjuvant chemotherapy for early-stage breast cancer:
the Eastern Cooperative Oncology Group experience. J Clin Oncol
1995;13:1557–63. Abstract.
Valagussa P,
Zambetti M, Biasi S, Moliterni A, Zucali R, Bonadonna G. Cardiac
effects following adjuvant chemotherapy and breast irradiation in
operable breast cancer. Ann Oncol 1994;5:209–16. Abstract.
van Dam FS,
Schagen SB, Muller MJ, Boogerd W, v d Wall E, Droogleever Fortuyn
ME, et al. Impairment of cognitive function in women receiving adjuvant
treatment for high-risk breast cancer: high-dose versus standard-dose
chemotherapy. J Natl Cancer Inst 1998;90:210–18. Abstract.
|
|

