|
 Home:
Educational Supplement: Appendix
Home:
Educational Supplement: Appendix
Is
Her-2/neu a Predictor of Anthracycline Utility
in Adjuvant Therapy? A Qualified Yes.
Peter
M. Ravdin, M.D., Ph.D.
The use of Her-2/neu
overexpression to select patients who might benefit from adjuvant
anthracyclines was first suggested in 1994. Now, 6 years later,
the use of Her-2 to guide adjuvant chemotherapy still remains a
matter of controversy. Nonetheless, there is some evidence that
Her-2 can and should be used for this purpose. It is therefore worthwhile
to examine the reasons why this evidence has fallen short of being
convincing, and why Her-2 overexpression has not yet become a standard
method to help select adjuvant therapy.
The principal
shortcoming of the evidence is that none of the adjuvant trials
whose results have been used to examine this question were designed
for that purpose. They were designed to answer treatment questions
only. Moreover, a two-arm trial becomes a four-arm trial when stratified
by a biomarker, and Her-2 is a particularly nettlesome biomarker
because it is overexpressed in only about 20 to 30 percent of cases.
As a result, the overexpressing subset is generally small. That
weakens the power of the studies.
How big would
an ideal trial be? Estimating the sample size as a two-arm trial
in node-positive patients with adjuvant anthracycline-based therapy
being twice as effective in Her-2 overexpressing patients, and about
20 to 30 percent of the patients overexpressing Her-2, the number
of patients required for the trial would be 2,000 to 3,000. Given
that the largest reported studies dealing with Her-2 in adjuvant
therapy involved about 1,000 patients, it is clear that most of
what we know is based on underpowered studies, many of them with
a power of 0.50 or less.
This would seem
to be an ideal area for a meta-analysis, but that would be somewhat
problematic because of another feature of the studies pertaining
to Her-2. Unlike treatment trials with well-defined standardized
endpoints (disease-free survival [DFS] and overall survival [OS]),
the endpoints (stratification) for using Her-2 are not well defined.
There was no uniformity in the trials in how Her-2 testing was done;
a number of different systems (based on percentage of cells staining,
stain intensity, and stain cellular location) were used to interpret
whether Her-2 was overexpressed by a given tumor. Thus, some of
the variation in the results of the studies may be due to simple
methodological differences.
It has been
argued that the mechanism by which Her-2 overexpression leads to
a sensitivity to adjuvant anthracyclines is unclear. The mechanism
is indeed rather obscure, with some preclinical systems suggesting
that it exists, and some finding just the opposite—that Her-2
overexpression may confer resistance to anthracyclines. Adding to
the confusion is the fact that Her-2 overexpression in patients
with metastatic disease is not unequivocally associated with response
to anthracyclines, and in some instances seems to have been associated
with resistance (Vincent-Salomon, Carton, Freneaux, et al. 2000;
Sjostrom, Krajewski, Franssila, et al. 1998; Jarvinen, Holli, Kuukasjarvi,
et al. 1998). These studies of metastatic disease were generally
small, however, and may be confounded by the biology of Her-2, which
in general is associated with unfavorable features (such as faster
cell proliferation) that may mask or counterbalance the benefits
of higher efficacy.
What is clear
is that arguments for the use of Her-2 in selecting adjuvant chemotherapy
are not as simple as the arguments for use of the estrogen receptor
(ER) in selecting adjuvant endocrine therapy. In the case of the
estrogen receptor, there are very clear molecular correlates, preclinical
system evidence, and supportive evidence in metastatic disease for
use of the ER. In addition, the ratio of response rates in metastatic
disease based on the estrogen receptor’s presence or absence
is 10:1. It is clear from the data that the usefulness of Her-2
in assessing the benefit of anthracyclines will be more modest.
Having conceded
these points, it is important to review how this HER-2 hypothesis
was developed and the consistency of the clinical trial evidence
that supports it. The initial report was from the CALGB, which analyzed
a trial in which node-positive breast cancer patients were randomized
between one of three different dose intensities of cyclophosphamide
and Adriamycin. Only in the Her-2 overexpressing patients did the
dose make a difference, and thus it appeared that Her-2 overexpression
identified a subset of patients who particularly benefited from
anthracyclines (Muss, Thor, Berry, et al., 1994).
The CALGB’s
own attempt to replicate those results has been controversial. The
second CALGB study used patients from the second half of the trial,
but the benefit was not as clear and did not reach statistical significance.
But by using elaborate statistical techniques and combining data
from the two halves of the study, the investigators were again able
to demonstrate that Her-2 overexpression identified a subset of
patients with anthracycline sensitivity (Thor, Berry, Budman, et
al., 1998). Those studies have inspired a number of other tests
of whether Her-2 overexpression identified a subset of patients
who were particularly likely to benefit from adjuvant therapy.
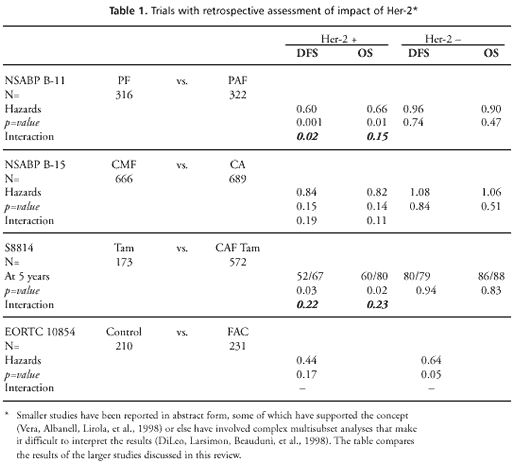
The first of
those studies used results from NSABP B-11. In that trial, patients
were randomized between melphalan and 5-fluorouracil (PF) or the
same drugs plus doxorubicin (PAF). Patients overexpressing Her-2
were the only subset for whom PAF resulted in a statistically significant
risk reduction, despite the fact that the subset was only about
half the size of the subset with low (normal) Her-2 expression.
The test for a statistically significant difference in risk reduction
between the subsets reached statistical significance for DFS and
showed a strong trend as a predictor of OS (Paik, Bryant, Park,
et al., 2000).
A second NSABP
study extended these observations. In this study, based on material
from NSABP B-15, tumor samples from the arms receiving CMF or CA
were analyzed. Again, the hazards of relapse and death were more
strongly reduced in the patient subset overexpressing Her-2 and
receiving CA. The test for interaction did not reach statistical
significance, but a trend toward greater benefit was seen (Paik,
Bryant, Tan-Chiu, et al., 1998).
The same trends
were seen in S8814, where postmenopausal ER+ patients were randomized
between tamoxifen and CAF plus tamoxifen (a 3 to 10 randomization).
Again, chemoendocrine therapy was superior only in the Her-2 overexpressing
patients, but once again the difference did not reach statistical
significance (Ravdin, Green, Albain, et al., 1998).
The last large
study to provide data for the present review was EORTC 10854, in
which node-negative premenopausal women were randomized to one perioperative
(immediately postoperative) cycle of CAF or to no further adjuvant
therapy. At 4 years of followup, a reduction in the hazard of relapse
was seen in both the Her-2 positive and Her-2 negative subsets,
but appeared larger in the Her-2 positive subset. Here too, the
difference did not reach statistical significance (Clahsen, van
de Velde, Duval, et al., 1998).
In summary,
a pattern is emerging in adjuvant studies showing that the Her-2
positive (overexpressing) subset derives greater benefit from adjuvant
therapy than the Her-2 negative subset. The low statistical power
of the four studies makes this effect difficult to demonstrate statistically,
but the studies do suggest that such an interaction exists.
References
Clahsen PC,
van de Velde CJ, Duval C, Pallud C, Mandard AM, Delobelle-Deroide
A, et al. p53 protein accumulation and response to adjuvant chemotherapy
in premenopausal women with node-negative early breast cancer. J
Clin Oncol. 1998; 16:470-9. Abstract.
DiLeo A, Larsimon
D, Beauduni M, et al. CMF or anthracycline-based adjuvant chemotherapy
for node-positive (N+) breast cancer (BC) patients (PTS): 4 year
results of a Belgian randomised clinical trial with predictive markers
analysis. Proc Am Soc Clin Oncol 1998; 18:258a. Abstract.
Jarvinen TA,
Holli K, Kuukasjarvi T, Isola JJ. Predictive value of topoisomerase
IIalpha and other prognostic factors for epirubicin chemotherapy
in advanced breast cancer. Bri Cancer 1998; 77:2267-73. Abstract.
Muss HB, Thor
AD, Berry DA, Kute T, Liu ET, Koerner F, et al. c-erbB-2 expression
and S-phase activity predict response to adjuvant therapy in women
with node-positive early breast cancer. Eng Medicine 1994; 330:1260-6.
Abstract.
Paik S, Bryant
J, Park C, Fisher B, Tan-Chiu E, Hyams D, et al. HER2 and choice
of adjuvant chemotherapy for invasive breast cancer. NSABP Protocol
B-15. Submitted 2000. Abstract.
Paik S, Bryant
J, Park C, Fisher B, Tan-Chiu E, Huams D, et al. erbB-2 and response
to doxorubicin in patients with axillary lymph node-positive, hormone
receptor-negative breast cancer. J Nat Cancer Inst 1998; 90:1361-70.
Abstract.
Ravdin P, Green
S, Albain K, et al. Initial report of the SWOG biological correlative
study of c-erbB-2 expression as a predictor of outcome in a trial
comparing adjuvant CAF T with tamoxifen alone. Proc Am Soc Clin
Onco 1998; 17:97a. Abstract.
Sjostrom J,
Krajewski S, Franssila K, Niskanen E, Wasenius VM, Nordling S, et
al. A multivariate analysis of tumour biological factors predicting
response to cytotoxic treatment in advanced breast cancer. Bri Cancer
1998; 78:812-5. Abstract.
Thor AD, Berry
DA, Budman DR, Muss HB, Kute T, Henderson IC, et al. erbB2, p53,
and efficacy of adjuvant therapy interactions in node-positive breast
cancer. Nat Cancer Inst 1998; 90: 1346-1360. Abstract.
Vera R, Albanell
J, Lirola JL, et al. HER2 overexpression as a predictor of survival
in a trial comparing adjuvant FAC and CMF in breast cancer. Proc
Am Soc Clin Oncol 1998; 18:265a. Vincent-Salomon A, Carton M, Freneaux
P, Palangie T, Beuzeboc P, Mouret E, et al. ERBB2 overexpression
in breast carcinomas: no positive correlation with complete pathological
response to preoperative high-dose anthracycline-based chemotherapy.
Eur Cancer 2000; 36:586-91. Abstract.
Vincent-Salomon A, Carton M, Freneaux P, Palangie T, Beuzeboc P,
Mouret E, et al. ERBB2 overexpression in breast carcinomas: no positive
correlation with complete pathological response to preoperative
high-dose anthracycline-based chemotherapy. Eur Cancer 2000; 36:586-91.
Abstract.
|
|

