|
 Home:
Educational Supplement: Appendix
Home:
Educational Supplement: Appendix
Duration
of Adjuvant Tamoxifen Therapy
John
L. Bryant, Ph.D.
Clinical trials
of adjuvant tamoxifen have convincingly demonstrated benefits for
patients with estrogen receptor (ER)-positive breast cancer, regardless
of nodal status, age, or menopausal status (EBCTCG, 1998; Fisher,
Dignam, Bryant, et al., 1996). But despite the large number of trials,
there is still uncertainty regarding the optimal duration of tamoxifen
therapy. This review will address comparisons of duration of tamoxifen
treatment up to about 5 years (about which considerable evidence
has been reported in the past decade), and conclude with a summary
of trials which have compared 5 years of treatment with longer durations
(for which the evidence is not extensive).
Durations
Up To 5 Years
Four trials
which have been reported in the past decade present direct comparisons
of the worth of about 5 years of tamoxifen to shorter durations.
Two Eastern Cooperative Oncology Group (ECOG) trials (E4181 and
E5181) compared 1 to 5 years of tamoxifen in post- and premenopausal
node-positive patients, respectively (Falkson, Gray, Wolberg, et
al., 1990; Tormey, Gray, Abeloff, et al., 1992). The Swedish Breast
Cancer Cooperative Group (SBCCG) and Cancer Research Campaign (CRC)
trials were larger studies comparing 2 to 5 years of treatment (SBCCG,
1996; CRC, 1996). A fifth trial (TAM-01), which was reported in
abstract form in 1997, is designed to compare 2 to 3 years duration
to 12 to 13 years, but the mean followup available as of the report
was 4.5 years, so that currently available data from this trial
might best be interpreted as comparing 2 to 3 years with 6 to 7
years (Delozier, Spielmann, Mace-Lesec’h, et al., 1997). All
five studies have reported statistically significant reductions
in event rates in favor of the longer duration. The three studies
comparing about 2 to at least 5 years (SBCCG, CRC, TAM-01) all estimated
a reduction in the event rate of about 20 percent. Only one (SBCCG)
demonstrated a statistically significant survival advantage for
the longer duration (18 percent reduction in mortality rate, SD
7 percent), with one other trial (CRC) also showing a modest but
nonsignificant survival advantage (11 percent reduction, SD 13 percent).
In light of the evidence for a reduced recurrence rate, it may be
reasonable to attribute this to the relatively short followup reported
to date, and to the well-known carryover effect of treatment with
tamoxifen (EBCTCG, 1998; Fisher, Dignam, Bryant, et al., 1996).
Indirect comparison trials of about 1, 2 or 5 years duration based
on the 1998 EBCTCG overview lead to a similar conclusion, since
the trends of a reduced recurrence rate and reduced mortality over
this time range were strongly significant.
Five Years
Treatment Versus Longer Duration
Three trials
comparing 5 years of tamoxifen to longer periods of time were first
reported in 1996. These included NSABP B-14, a double-blind comparison
of 5 additional years of tamoxifen versus placebo in 1,172 node-negative
women who had completed 5 years of initial treatment disease-free;
the Scottish trial, in which 342 predominately node-negative women
were assigned to indefinite tamoxifen or observation following the
completion of 5 years of tamoxifen disease-free; and ECOG E4181/E5181,
in which 87 postmenopausal node-positive women from E4181 and 107
premenopausal node-positive women from E5181 who were disease-free
at 5 years were randomized to either indefinite tamoxifen or observation.
All patients in B-14 were ER-positive >10 fmol/mg protein), compared
to 73 percent in the ECOG trial. In the Scottish trial, 39 percent
of the patients had tumors with > 20 fmol/mg, 22 percent had
< 19 fmol/mg, and 39 percent were not assayed. The NSABP data
have recently been updated; the Scottish and ECOG data will be updated
at the EBCTCG meeting in 2000.
After a median
followup from re-randomization of 6.75 years, the B-14 data indicate
no advantage for continued tamoxifen, and in fact trend in the opposite
direction. Disease-free survival (DFS) 7 years following re-randomization
was 82 percent for placebo patients and 78 percent for those receiving
more than 5 years of tamoxifen (HR=1.3, p=0.03). Overall survival
was 94 percent for placebo patients and 91 percent for tamoxifen
patients (39 versus 57 deaths, HR=1.5, p=0.07). The distribution
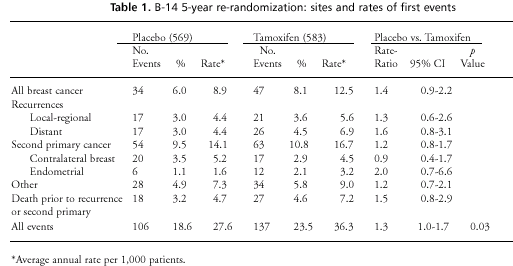
of first events is shown in table 1. Events in the primary NSABP
DFS analysis included recurrence, contralateral breast cancer (CBC),
second primary cancer, and death. Table 1 shows that only about
one-half (118 of 243) of all first events were “breast cancer
related” (i.e., recurrences or CBC). To facilitate comparison
with results of the ECOG trial described below, the comparison of
treatment arms was restricted to only first recurrences and CBC.
In terms of this endpoint the treatment difference was nonsignificant
(HR=1.21, p=0.31), although the trend was still suggestive of a
disadvantage for continued tamoxifen.
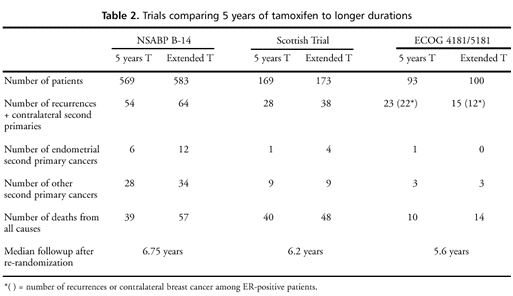
The NSABP data
are compared to the Scottish and ECOG data in table 2. Findings
to date from the Scottish trial are similar to those of B-14. A
nonsignificant detriment was reported in the analysis of time to
first recurrence, CBC, or death (HR=1.27, 95 percent CI 0.87–1.85)
as well as overall survival (40 deaths versus 48). As in the NSABP
trial, there were more endometrial cancers in those patients receiving
tamoxifen. The published results from ECOG 4181/5181 are somewhat
more positive. A nonsignificant advantage was seen for extended
tamoxifen in terms of time to first recurrence or CBC (23 versus
15 events; recurrence-free survival at 5 years after re-randomization
was 85 percent for those continuing tamoxifen versus 73 percent
for those stopping at 5 years, p=0.10). In a secondary analysis
restricted to patients who were ER-positive, this comparison became
significant (22 versus 12 events, p=0.014). There was, however,
no evidence of any survival benefit for continued tamoxifen (10
deaths in patients receiving 5 years of treatment versus 14 among
patients receiving extended tamoxifen, p=0.52).
In aggregate,
there have been more reported recurrences and CBCs (117 versus 105)
and deaths (119 versus 89) in patients randomized to continue tamoxifen
than to those who stopped treatment. The apparent difference in
the results of the ECOG trial and the other two studies may be due
to chance (a crude heterogeneity test for between-trial differences
based on recurrence counts is suggestive but not significant, p=0.09).
Alternatively, it has been suggested that the optimal duration of
treatment may differ for node-negative and node-positive patients
(Tormey, Gray, Falkson, 1996). This may be so even if the risk reduction
associated with breast cancer was independent of nodal status (as
suggested by the EBCTCG overview data), since the recurrence rate
in node-negative patients is considerably less than that in node-positive
patients, even after 5 disease-free years (Tormey, Gray, Falkson,
1996). Long-term treatment with tamoxifen is associated with certain
serious risks, most notably thromboembolic disease, endometrial
cancer, and, possibly, stroke (EBCTCG, 1998; Fisher, Costantino,
Redmond, et al., 1994; Fisher, Costantino, Wickerham, et al., 1998).
Therefore, even if it could be demonstrated that continued tamoxifen
were efficacious, its risk/benefit ratio for node-negative women
would be higher than for node-positive women (and higher for older
women compared to younger).
Active Trials
Testing Durations Beyond 5 Years
Both the aTTom
(CRC Trials Unit, no date) and the ATLAS (Clinical Trials Service
Unit, 1995) trials are now randomizing patients after 5 years of
successful treatment with tamoxifen, either to stop the treatment
or to continue it for an additional 5 years (Peto, personal communication).
There are also three currently active trials in which hormone-responsive
postmenopausal patients who have been successfully treated with
tamoxifen for about 5 years are randomized to receive an additional
2 to 5 years of treatment with an aromatase inhibitor or a placebo.
These include NCIC/EORTC/IBCSG/NCCTG/ECOG/ SWOG/CALGB MA 17 (Piccart,
Goldhirsch, no date) (letrozole); NSABP B-33 (NSABP, 2000) (exemestane);
and ABCSG Study 6A (Piccart, Goldhirsch, no date) (anastrozole).
Conclusions
There is strong
evidence that 5 years of tamoxifen is superior to 2 to 3 years,
at least in terms of delaying recurrence. Results up to now from
trials comparing 5 to more than 5 years fail to indicate an additional
advantage for continued tamoxifen, but the evidence is insufficient
to achieve a satisfactory level of consensus, and continued enrollment
of properly consented patients in long-term tamoxifen trials is
appropriate. Alternatively, patients who have remained disease-free
for extended periods of time on tamoxifen should be strongly considered
for participation in clinical trials of other hormonal interventions,
including aromatase inhibitors or selective estrogen receptor modulators
(SERMs). Outside a clinical trials setting, adjuvant treatment with
tamoxifen should be limited to 5 years duration until such time
as additional data become available to definitively resolve the
issue of tamoxifen duration.
References
Clinical Trial
Service Unit, Radcliffe Infirmary, Oxford. Adjuvant tamoxifen longer
against shorter (ATLAS). Protocol. April, 1995. ATLAS Office, Clinical
Trials Service Unit. Radcliffe Infirmary, Oxford OX2 6HE, U.K.
Abstract.
CRC Trials Unit,
Birmingham. Adjuvant tamoxifen treatment offer more? (aTTom). Protocol.
CRC Trials unit, Clinical Research Block, Queen Elizabeth Hospital,
Birmingham B15 2TH, U.K. Abstract.
Current Trials
Working Party of the Cancer Research Campaign (CRC) Breast Cancer
Trials Group. Preliminary results from the Cancer Research Campaign
trial evaluating tamoxifen duration in women aged fifty years or
older with breast cancer. J Natl Cancer Inst 1996;88:1834-9.
Abstract.
Delozier T,
Spielmann M, Mace-Lesec’h J, Janvier M, Luboinski M, Asselain
B, et al. Short-term versus lifelong adjuvant tamoxifen in early
breast cancer (EBC): a randomized trial (TAM-01). [abstract]. Proc
Am Soc Clin Oncol 1997;16. Abstract.
Early Breast
Cancer Trialists’ Collaborative Group (EBCTCG). Tamoxifen for
early breast cancer: an overview of the randomized trials. Lancet
1998;351:1451-67. Abstract.
Falkson HC,
Gray R, Wolberg WH, Gillchrist KW, Harris JE, Tormey DC, et al.
Adjuvant trial of 12 cycles of CMFPT followed by observation or
continuous tamoxifen versus four cycles of CMFPT in postmenopausal
women with breast cancer: An Eastern Cooperative Oncology Group
phase III study [published erratum appears in J Clin Oncol 1990;8:1603].
J Clin Oncol 1990;8:599-607. Abstract.
Fisher B, Costantino
JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial
cancer in tamoxifen-treated breast cancer patients: findings from
the National Surgical Adjuvant Breast and Bowel Project (NSABP)
B-14. J Natl Cancer Inst 1994;86:527-37. Abstract.
Fisher B, Costantino
JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen
for prevention of breast cancer: report of the National Surgical
Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst
1998;90:1371-88. Abstract.
Fisher B, Dignam
J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, et al. Five versus
more than five years of tamoxifen therapy for breast cancer patients
with negative lymph nodes and estrogen receptor-positive tumors.
J Natl Cancer Inst 1996;88:1529-42. Abstract.
Fisher B, Dignam
J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen
for node-negative breast cancer: updated findings. J Natl Cancer
Inst [submitted]. Abstract.
National Surgical
Adjuvant Breast and Bowel Project. NSABP B-33: A randomized, placebo-controlled,
double-blind trial evaluating the effect of exemestane in clinical
stage cT1-3 cN0-1 M0 postmenopausal breast cancer patients completing
at least five years of tamoxifen therapy. Protocol. 2000. Pittsburgh:
NSABP. Peto R, personal communication. Abstract.
Piccart M, Goldhirsch
A, on behalf of the International Breast Cancer Study Group. Biganzoli
L and Straehle C, editors. An overview of recent and ongoing adjuvant
clinical trials for breast cancer, second ed. No Abstract Available.
Stewart HJ,
Forrest AP, Everington D, McDonald CC, Dewar JA, Hawkins RA, et
al. Randomised comparison of 5 years of adjuvant tamoxifen with
continuous therapy for operable breast cancer. The Scottish Cancer
Trials Breast Group. Br J Cancer 1996;74:297-9. Abstract.
Swedish Breast
Cancer Cooperative Group (SBCCG). Randomized trial of two versus
five years of adjuvant tamoxifen for postmenopausal early stage
breast cancer. J Natl Cancer Inst 1996;88:1543-9. Abstract.
Tormey DC, Gray
R, Abeloff MD, Roseman DL, Gilchrist KW, Barylak EJ, et al. Adjuvant
therapy with a doxorubicin regimen and long-term tamoxifen in premenopausal
breast cancer patients: An Eastern Cooperative Oncology Group trial.
J Clin Oncol 1992;10:1848-56. Abstract.
Tormey DC, Gray
R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond
five years in patients with lymph node-positive breast cancer. Eastern
Cooperative Oncology Group. J Natl Cancer Inst 1996;88:1828-33.
Abstract.
|
|

