| You are here: Home: BCU 7|2003: Richard
M Elledge, MD

Side effects of fulvestrant versus anastrozole
One of the adverse events evaluated in these trials was thromboembolic
events. From our experience with the aromatase inhibitors, we would
not expect to see an increase in thromboembolic events with anastrozole,
and in both trials of anastrozole versus fulvestrant, the number
of thromboembolic events in the two arms was virtually identical.
We did not see evidence in these trials that fulvestrant causes
more thrombosis. Because this agent is a steroid molecule with
many similarities to estrogen, this was somewhat of a concern,
but I was glad to see no evidence that it is thrombogenic.
Trial of fulvestrant versus tamoxifen as first-line
therapy
Much to our surprise, this trial did not demonstrate that fulvestrant
was superior to tamoxifen in the first-line setting. Extrapolating
what we know from previous trials of fulvestrant versus anastrozole,
and of anastrozole versus tamoxifen, we predicted that fulvestrant
would be better than tamoxifen. However, in the study we just didn't
see it.
Some have suggested that the dose of fulvestrant was inadequate.
While I believe this should be explored, I'm not entirely convinced
it is the reason. Another possibility relates to the fact that
most patients in the second-line study had been treated with tamoxifen
or were coming straight off of tamoxifen. This may have somehow
altered the phenotype, perhaps causing fulvestrant to work better
in the second-line trial, as opposed to treatment-naïve tumors
or those that have not been exposed to tamoxifen recently. After
reviewing the data, the reason the first-line trial didn't demonstrate
fulvestrant to be superior to tamoxifen is still not clear.
Clinical experience with fulvestrant
In my clinical experience, fulvestrant is very easy to administer
and extremely well-tolerated. My patients have not had any problems
with the intramuscular injection. One might assume that a pill
is more convenient therapy for a patient than an injection, but
that is not necessarily so. Convenience is an individual choice.
Some patients would rather receive a shot once a month than take
a pill every day.
Not only has fulvestrant been exceptionally well-tolerated, I've
seen responses in heavily pretreated patients. Fulvestrant also
works after multiple endocrine failures, including on tamoxifen
and the aromatase inhibitors, even in a third- or fourth-line setting.
We now have a very well-tolerated endocrine agent to add to our
armamentarium in the metastatic setting.
Interactions between growth factor pathways and
the estrogen receptor
Possible interaction between polypeptide growth factor pathways
and the estrogen receptor might present opportunities for therapy.
Estrogen receptor biology has evolved over the last several years
in terms of interaction between the estrogen receptor and coactivators
and corepressors. These interactions may determine the final output
of the estrogen receptor.
In addition, the estrogen receptor may be important in other ways
beyond the classical binding to DNA and turning on estrogen-responsive
genes through estrogen response elements. Estrogen receptor also
binds to other types of transcription factors and helps regulate
genes. There's also a growing awareness that estrogen receptor
exists in the cell membrane and may be able to activate other growth
factor pathways directly by interacting with the receptor via intermediate
signaling molecules.
If we can block some of this activation - either the estrogen
receptor activating other growth factor pathways or growth factor
pathways activating the estrogen receptor - the clinical implication
is that combined therapies may be better than monotherapy.
The therapies optimal for combination are those that block tyrosine
kinase activity, such as gefitinib and trastuzumab. A fairly striking
delay in tumor growth has been seen when gefitinib has been combined
with tamoxifen or estrogen withdrawal in HER2-nonoverexpressing
tumors. It would be interesting to see combination trials with
the aromatase inhibitors and with fulvestrant.
Proposed NSABP trial of fulvestrant, anastrozole
and gefitinib
I proposed a trial to the NSABP that would look at a combination
of three agents - fulvestrant, anastrozole and gefitinib. The trial
will utilize fulvestrant to downregulate the estrogen receptor.
Anastrozole will then downregulate the ligand in the system, and
gefitinib will decrease any crosstalk that may activate the estrogen
receptor through other pathways.
The proposed NSABP trial will be a one-armed, Phase II study in
60 patients. The patients will be postmenopausal women with hormone-responsive
tumors greater than three centimeters in size. The three drugs
will be given in combination in a neoadjuvant fashion for four
months. The therapeutic endpoint will be tumor regression and pathologic
findings at surgery.
We will also evaluate molecular endpoints. We plan to do core
needle biopsies before the patient goes on study and again at two
weeks, and we will obtain tissue at the time of surgery. We will
study molecular changes within the tissue, specifically ER levels,
AKT and MAP kinase levels and phosphorylation status.
Side effects and toxicity shouldn't be a problem. The only problem
I can foresee is a possible skin rash from gefitinib, but we reduced
the dose to the 250-mg level. Significant skin rash was reported
in the breast cancer trial presented last year in San Antonio,
but the dose used in that study was 500 milligrams.
There may be some skepticism about combining hormonal therapy
after the disappointing results from the combination arm of the
ATAC trial. However, the meta-analysis of three randomized studies
evaluating tamoxifen plus an LHRH agonist versus an LHRH agonist
alone in premenopausal patients shows not only an advantage in
response rate and time-to-treatment failure, but also a survival
advantage for combination hormonal therapies.
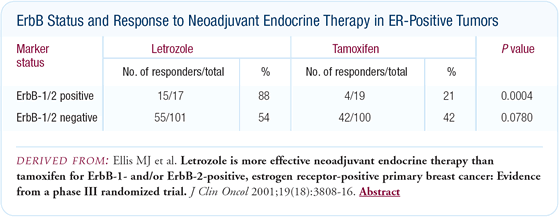
Hormone sensitivity of HER2-positive, ER-positive
tumors
A good deal of laboratory evidence shows that HER2-positive, ER-positive
tumors are less responsive to tamoxifen than HER2-negative, ER-positive
tumors. This issue becomes less clear in the clinic. When both
the ER assay and the HER2 assay are done correctly, I believe the
proportion of patients with HER2-positive, ER-positive tumors is
actually quite low - in the range of five percent to 10 percent
of all patients. With such a small subset, it is difficult to perform
adequately powered studies to provide a clear answer regarding
hormone sensitivity.
Another confounding element is that the ER content in HER2-positive,
ER-positive tumors, is about one-half to one-third of the ER content
in the HER2-negative tumors. Some of this "resistance" may therefore
be a function of lower or absent ER. Clinically it is not clear
to me whether HER2 overexpression causes tamoxifen resistance.
The balance of emerging data does point to a possible modest resistance.
Defining ER-positivity
European studies have shown that approximately 20 percent of ER
assays are false negatives when compared to a reference lab. Estrogen
receptor testing is not standardized in the United States or Europe,
and this leads to a great deal of suboptimal treatment and misunderstanding
of breast cancer biology. For years, we thought that some ER-negative
patients responded to hormonal therapy; however, I believe this
was merely a result of poor assay methodology.
Part of the problem with these assays is technical, and part is
in the interpretation. On the technical side, pathologists are
just not used to performing immunohisto-chemistry. The technique
is not standardized. Many pathologists come up with their own methods
and only do a few cases a week. This lack of standardization and
experience causes technical issues and false-negative results.
Interpretation of assay results is a problem in terms of both staining
and cutoff values. Many laboratories have established a cutoff
that is too high and have labeled tumors with ER as being ER-negative.
We have shown in multiple studies in the advanced-disease setting,
the adjuvant setting and the DCIS setting that tumors with more
than one percent of cells staining positive are hormone responsive,
while tumors with less than one percent of cells staining don't
appear to benefit from endocrine therapy.
I believe that medical oncologists often just assume the pathologist
is correct. When we started closely reviewing results in our tumor
board, it was obvious that there were big problems. Clinicians
can insist on having tumors processed in a central laboratory that
has a high volume that uses a clinically validated methodology.
Sequencing chemotherapy in the metastatic setting
In terms of sequencing chemotherapy in the metastatic setting,
I generally start with an anthracycline in patients who did not
receive them in the adjuvant setting. Otherwise, I usually begin
with a taxane. Capecitabine is my next chemotherapy choice after
anthracyclines and taxanes.
Especially in elderly or frail patients, I always bring capecitabine
into the equation. Not only is it oral, but it is also associated
with a good quality of life if the dose is somewhat attenuated
and we monitor for hand-foot syndrome.
I usually start capecitabine as a single agent at 2,000 mg/m2
for three to five cycles and then a rest. I do not routinely use
it with docetaxel, though I recognize that a number of people do,
and that there are some good reasons to do so in certain conditions.
Page 2 of 2
Previous | Select publications
|
