|
You are here: Home: BCU Surgeons |2002: Patterns of Care Study

Commentary
The primary rationale for utilizing tamoxifen in high-risk women
in clinical trials, such as NSABP P-1, was the reduction in contralateral
breast cancer observed with adjuvant tamoxifen in patients with
invasive breast cancer. The preliminary results of the ATAC trial
demonstrated 56% fewer second breast cancers in women randomized
to anastrozole compared to tamoxifen. Based on these early findings,
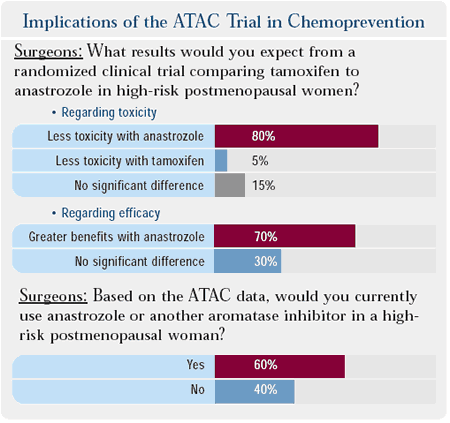
most surgeons surveyed believe that a randomized trial comparing
anastrozole to tamoxifen in high-risk postmenopausal women would
demonstrate both greater efficacy and less toxicity for anastrozole.
Sixty percent of surgeons would use anastrozole in these women at
the present time, for which there is no FDA indication, but breast
cancer researchers almost uniformly believe that aromatase inhibitors
should only be utilized in high-risk patients as part of a clinical
trial. In the United Kingdom, a massive trial is being planned to
evaluate the use of anastrozole in high-risk patients. The final
design of this IBIS II trial is awaiting the presentation of IBIS
I study results comparing tamoxifen to placebo.
Return
|
|
