| You
are here: Home: BCU 4|2001: Section 4
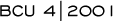
Section 4
Randomized Trial of Docetaxel-Capecitabine
versus Docetaxel
RESULTS
The study was
conducted in 16 countries at 25 different sites,and a crossover
was not built into it, because not all of the institutions and countries
had access to capecitabine. So it was not designed as a crossover
trial. The question being addressed was combination therapy versus
full-dose docetaxel monotherapy.
It turned out
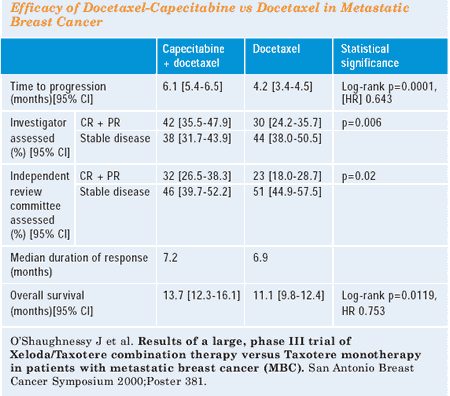
that 15% of women receiving single-agent docetaxel crossed over
to receive capecitabine. Two-thirds of the patients went on to receive
further cytotoxic chemotherapy — 25% of them received additional
vinorelbine, for example. So, women were treated appropriately with
what sounds like good standard therapy afterwards,and there were
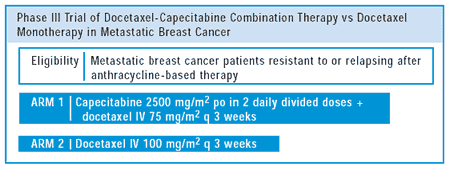
no imbalances there. What was seen in this trial was that the combination
led to a superior, statistically significant, objective response
rate and a 33% improvement in median time to progression. Most significantly,
a 25% relative improvement in median overall survival was observed.
Importantly, the survival curves pulled apart very early —
so it became very clear earlier in the study that more women were
living with the combination compared to the single agent.
After giving
this a lot of thought, I have concluded that there is at least a
reasonable possibility that the biochemical synergism between these
agents was largely responsible for the survival advantage. This
was not front-line therapy for metastatic breast cancer; it was
fairly late-line therapy,and it was a very heavily tumor-burdened
population of women. And the time to progression and survival curves
separated early.
I am just not
sure that you would have captured the same degree of survival advantage
in this late-line therapy situation by giving the drugs sequentially.
That’s my personal opinion, but we simply don’t know.
—Joyce
O’Shaughnessy, MD
Page
2 of 4
Back | Next
|
