|
You are here: Home: BCU 2|2001:
Taxanes in the Adjuvant
Setting: Why Not Yet?
Martine J.
Piccart, M.D., Ph.D., Caroline Lohrisch, and Luc Duchateau
Adjuvant chemotherapy
with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) and
anthracycline-based regimens is associated with significant reductions
in breast cancer mortality (EBCTCG, 1998). Numerous other treatment
strategies have been explored in attempts to further improve survival,
including newer anticancer drugs with demonstrated activity in the
metastatic setting, such as the taxanes (T). So far, two randomized
trials of the adjuvant taxane paclitaxel have been reported. An
Intergroup trial (Henderson, Berry, Demetri, et al., 1998) compared
the efficacy of 4 AC with 4 AC followed by 4 paclitaxel (AC T)
in node-positive cancer, while the M.D. Anderson Cancer Center trial
compared 8 FAC to 4 T followed by 4 FAC (T T)
in node-positive cancer, while the M.D. Anderson Cancer Center trial
compared 8 FAC to 4 T followed by 4 FAC (T FAC)
in node-negative and node-positive breast cancer (Thomas, Buzdar,
Theriault, et al., 2000). The positive results in the Intergroup
trial (overall survival [OS] 95 percent versus 97 percent, p=0.04,
and disease-free survival [DFS] 86 percent versus 90 percent, p=0.0008)
led to approval by the Food and Drug Administration (FDA) of adjuvant
paclitaxel for node-positive breast cancer. The absolute survival
difference, however, is quite small, just below the 5 percent significance
level. The Anderson trial, reported subsequently, showed nonsignificant
results (DFS 81.5 percent versus 85.2 percent, p=0.2, and 15 deaths
versus 13 deaths for T FAC)
in node-negative and node-positive breast cancer (Thomas, Buzdar,
Theriault, et al., 2000). The positive results in the Intergroup
trial (overall survival [OS] 95 percent versus 97 percent, p=0.04,
and disease-free survival [DFS] 86 percent versus 90 percent, p=0.0008)
led to approval by the Food and Drug Administration (FDA) of adjuvant
paclitaxel for node-positive breast cancer. The absolute survival
difference, however, is quite small, just below the 5 percent significance
level. The Anderson trial, reported subsequently, showed nonsignificant
results (DFS 81.5 percent versus 85.2 percent, p=0.2, and 15 deaths
versus 13 deaths for T FAC
and FAC, respectively). FAC
and FAC, respectively).
The Intergroup
and Anderson trials had important differences in design and patient
eligibility. The proportion of patients in the Anderson trial with
none, 1 to 3, or more than 3 positive nodes was approximately one-third
each. This group therefore had a slightly better overall prognosis
than the Intergroup population, in which no patients were node-negative
and the proportion with 1 to 3 and more than 3 nodes was approximately
even. More importantly, the two trials differed substantially with
respect to total sample size and median followup. The Intergroup
trial involved 3,170 patients, versus 524 in the Anderson trial,
while the Anderson had more than double the median followup (18
months versus 43 months). In using a time-to-event analysis, the
power of a trial to detect a significant effect rests primarily
on the number of events. Thus, it is completely plausible that the
Intergroup trial demonstrated a significant difference, given its
approximately 374 events (DFS) and 125 deaths at 18 months, while
the Anderson trial, with 75 events and 28 deaths, does not, despite
longer followup. To what extent these differences influence the
apparently disparate results of these two studies is not clear.
Nevertheless, the conclusion must be that the results of the Anderson
trial neither help nor hinder the case for adjuvant paclitaxel.
There are, however, a number of reasons to support the view that
the FDA may have made a hasty decision. Note that, after reviewing
the same data, the European Regulatory Agency decided not to approve
paclitaxel for adjuvant therapy.
Confidence in
any treatment depends on whether its value is consistently reproducible.
The general view within the scientific community is that a single
randomized trial does not constitute sufficient level I evidence,
no matter how compelling the results. This view arises from repeated
observation that a particular regimen's superior results cannot
always be duplicated in subsequent trials. Despite that well-known
principle, the FDA seems to have been persuaded by the results of
only one trial. Given that numerous ongoing randomized trials of
taxanes will be reported in the next few years and that the absolute
survival difference reported by the Intergroup trial was modest
(2 percent), it would have been more prudent to await corroborative
evidence before approving the routine use of adjuvant taxanes.
Design Limitations
of Intergroup Trial
Furthermore,
the Intergroup trial had design limitations, and it is unclear to
what extent those limitations accounted for the treatment effect.
A major potential confounder in this trial was the duration of therapy,
which was 12 weeks longer in the taxol-containing arm. Several trials
have demonstrated that duration of therapy may indeed influence
outcome, but reports on the trials do not deal with the relative
importance of duration of therapy and cumulative dose. Both of these
factors may have biased the results in favor of the AC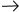 T
arm, since the cumulative dose of doxorubicin in one-third of the
study population was 240 mg/M2, which may be suboptimal. T
arm, since the cumulative dose of doxorubicin in one-third of the
study population was 240 mg/M2, which may be suboptimal.
The Oxford
overview reported that anthracycline therapy may be associated with
a small survival advantage over CMF (EBCTCG, 1998), but other studies
have failed to demonstrate that anthracycline therapy beyond a threshold
dose improves survival. There does seem to be a dose-response relationship
below that threshold, with compromised efficacy of anthracyclines
(Wood, Budman, Korzun, et al., 1994; Bonneterre, Roche, Bremond,
et al., 1998). Thus, we are faced with the question of whether the
cumulative dose of doxorubicin in 4 AC (240 mg/M2) was below this
threshold, making it as effective as CMF but less effective than
it could be. If the answer is yes, it may explain the apparent discrepancy
in the results of NSABP B-15 (equivalence for 6 CMF and 4 AC 240
mg/M2 doxorubicin total dose), the National Cancer Institute of
Canada (NCIC) comparison of CEF and CMF (CEF giving a 7 percent
superior OS, 720 mg/M2 epirubicin total dose), and the Intergroup
trial of CAF versus CMF (CAF giving a 2 percent superior OS) (Hutchins,
Green, Ravdin, et al., 1998; Levine, Bramwell, Pritchard, et al.,
1998). Although 4 AC (doxorubicin 60 mg/M2/cycle) is a standard
adjuvant regimen in North America, many European clinicians give
several cycles of CMF following 4 AC, or a higher total dose of
anthracyclines, such as can be found in CAF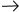 FAC
and CEF FAC
and CEF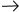 FEC
regimens. FEC
regimens.
Another major
concern with the Intergroup trial is the immaturity of the data.
The initial results were reported 8 months after the accrual of
the last patient (3-year accrual period), based on a preplanned
interim analysis of 450 events. These significant results cannot
be extrapolated to later points in time unless one assumes a constant
proportional hazards model. If that assumption is not correct, however,
more mature results could show a "reversal of fortune," as did the
EORTC neoadjuvant breast cancer study (Sylvester, Bartelink, Rubens,
1994). An interim analysis demonstrated significant superiority
for chemotherapy, but reanalysis 2.5 years after the last patient
was accrued reversed the favorable outcome of hormonal therapy,
thus demonstrating the limitations of analyses based on early data.
The Intergroup analysis, carried out when, on average, 96 percent
of the patients were still alive and 88 percent were disease-free,
is no exception. One could even call the more recent analysis (at
30 months median followup) immature, given the recurrence rate in
the control arm of 22 percent, since a higher final recurrence rate
for a node-positive population treated with anthracyclines would
be expected, based on previous trials (Fisher, Brown, Dimitrov,
et al., 1990; Levine, Bramwell, Pritchard, et al., 1998).
Finally, there
is the issue of whether all patients in the study derived equal
benefit from the treatment. A subset analysis suggests that only
patients with hormone receptor (HR)-negative tumors (one third of
the study population) benefited from the addition of tamoxifen.
For the 2,066 HR-positive patients, the hazard ratio for recurrence
was 0.92 (95 percent CI 0.73-1.16) for AC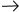 T
versus AC, while for HR-negative patients it was 0.68 (95 percent
CI 0.55-0.85). A similar trend was observed in the Anderson trial:
58 percent of the population was HR-positive, and although not statistically
significant, the absolute difference in DFS for FAC versus T T
versus AC, while for HR-negative patients it was 0.68 (95 percent
CI 0.55-0.85). A similar trend was observed in the Anderson trial:
58 percent of the population was HR-positive, and although not statistically
significant, the absolute difference in DFS for FAC versus T FAC
was 3 percent for HR-positive patients and 5 percent for HR-negative
patients. There are several potential explanations for these findings.
Either the baseline risk for HR-positive tumors is lower and therefore
a benefit of tamoxifen is more difficult to demonstrate or does
not exist, or the baseline risk is sufficiently lowered by AC and
tamoxifen that the added benefit of tamoxifen, if it exists, cannot
be demonstrated with this sample size and followup period. Another
explanation may be that recurrences in the HR-positive population
occur later, and a benefit may only become apparent with longer
followup. Regardless of the reason, this subset analysis supports
the contention that, based on the available evidence, sweeping generalizations
about the value of adjuvant paclitaxel are premature. FAC
was 3 percent for HR-positive patients and 5 percent for HR-negative
patients. There are several potential explanations for these findings.
Either the baseline risk for HR-positive tumors is lower and therefore
a benefit of tamoxifen is more difficult to demonstrate or does
not exist, or the baseline risk is sufficiently lowered by AC and
tamoxifen that the added benefit of tamoxifen, if it exists, cannot
be demonstrated with this sample size and followup period. Another
explanation may be that recurrences in the HR-positive population
occur later, and a benefit may only become apparent with longer
followup. Regardless of the reason, this subset analysis supports
the contention that, based on the available evidence, sweeping generalizations
about the value of adjuvant paclitaxel are premature.
Unsettled
Issues
Despite the
enormous strides made in adjuvant chemotherapy for breast cancer
over the last 20 years, there are a number of unsettled issues.
The NCIC trial faces the ambitious task of exploring the relative
importance of cumulative dose, dose density, and noncross-resistant
drugs added to anthracycline-based chemotherapy. The design calls
for randomization between three arms: 6 FEC (Levine regimen), 4
AC followed by 4 T, and 6 EC every 2 weeks with G-CSF followed by
4 T. If the results were available now, we might be able to put
to rest many of our reservations about the Intergroup trial, which
leave us a little unsettled about the long-term reliability and
generalizability of its results, regardless of how promising they
may appear. Unfortunately, the results are not available, and the
finding of nonsignificance in the Anderson study amplifies the uncertainty.
It is necessary to wait for future results of ongoing trials before
pronouncing judgment on the value of taxanes in the adjuvant setting.
It is also necessary
to better define the population most likely to benefit from therapies
of longer duration, intensification, and multiple regimens. It no
longer is reasonable to judge all breast cancer patients as having
equal probability of benefit from a given therapy. That was a paradigm
that worked well when adjuvant chemotherapy for breast cancer was
in its infancy and little was known about the molecular heterogeneity
of breast cancer. It is now of critical importance to design trials
with the aid of molecular tumor profiles with potential predictive
value to prospectively identify the subgroup most likely to benefit
from the addition to therapy of taxanes and other new drugs. This
process has begun with the EORTC-Breast Cancer Cooperative Group
trial in locally advanced breast cancerŅan attempt to examine the
predictive value of p53 mutations in response to taxane chemotherapy.
It is to be
hoped that the early promise of taxanes in adjuvant treatment of
breast cancer will be confirmed, since there are few encouraging
alternatives at this time. It is important to realize, however,
that the data we have now only support their potential. Further
followup, and trials that corroborate the results of the Intergroup
trial of AC versus AC¨T, are essential to define the value of taxanes
in early breast cancer.
References
Bonneterre J,
Roche H, Bremond A, et al. Results of a randomized trial of adjuvant
chemotherapy with FEC 50 vs FEC 100 in high risk node-positive breast
cancer patients. [abstract]. Proc Am Soc Clin Oncol 1998;17:124a.
Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Polychemotherapy
for early breast cancer: an overview of the randomised trials. Lancet
1998;352:930-42.
Fisher B, Brown
AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, et al. Two
months of doxorubicin-cyclophosphamide with and without interval
reinduction therapy compared with 6 months of cyclophosphamide,
methotrexate, and fluorouracil in positive node breast cancer patients
with tamoxifen-nonresponsive tumors: results from the National Surgical
Adjuvant Breast and Bowel Project B-15. J Clin Oncol 1990;8:1483-96.
Henderson IC,
Berry D, Demetri G, et al. Improved disease-free and overall survival
from the addition of sequential paclitaxel but not from the escalation
of doxorubicin dose level in the adjuvant chemotherapy of patients
with node-positive primary breast cancer. [abstract]. Proc Am Soc
Clin Oncol 1998;17:101a.
Hutchins LF,
Green S, Ravdin PM, et al. CMF versus CAF with and without tamoxifen
in high-risk node-negative breast cancer patients and a natural
history follow-up study in low-risk node-negative patients: first
results of Intergroup trial INT 0102. [abstract]. Proc Am Soc Clin
Oncol 1998;17:1a.
Levine MN, Bramwell
VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zabra H, et al. Randomized
trial of intensive cyclophosphamide, epirubicin, and fluorouracil
chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil
in premenopausal women with node-positive breast cancer. J Clin
Oncol 1998;16:2651-8
. Sylvester
R, Bartelink H, Rubens R. A reversal of fortune: practical problems
in the monitoring and interpretation of an EORTC breast cancer trial.
Stat Med 1994;13:1329-35.
Thomas E, Buzdar
A, Theriault R, et al. Role of paclitaxel in adjuvant therapy of
operable breast cancer: preliminary results of prospective randomized
clinical trial. [abstract]. Proc Am Soc Clin Oncol 2000;19:74a.
Wood WC, Budman
DR, Korzun AH, Cooper MR, Younger J, Hart RD, et al. Dose and dose
intensity of adjuvant chemotherapy for stage II, node-positive breast
carcinoma. N Engl J Med 1994;330:1253-9.*
|
|
